Amphetamine and methamphetamine dependence has become a substantial social and public health issue in the United Kingdom and Australia, and it has grown into a major illicit drug problem throughout the United States
(1) . In Finland, amphetamine is currently the most common illegal drug used intravenously, and the collateral problems associated with intravenous amphetamine use (e.g., unemployment, violence, crime, mortality, and HIV and other infections) have become a major national concern. Although cognitive behavior interventions, manualized treatment with cognitive behavior therapy, family education, support, and counseling may give some benefit
(2), there has been no evidence from randomized controlled trials using an intention-to-treat approach on the efficacy of any psychosocial treatment in decreasing intravenous amphetamine use.
While methadone and buprenorphine have proven highly effective substitute medications for opioid dependence
(3), no pharmacological treatment has been found thus far for amphetamine dependence
(4) . Partial dopamine agonists such as aripiprazole are considered to be promising medications for addiction, since they are supposed to balance and restore normal function of the mesolimbic dopamine system
(5) . Observational studies, nonrandomized trials, and one randomized study without a placebo arm have suggested that oral dextroamphetamine may be used to replace illicit intravenous amphetamine use
(4), which suggests that oral methylphenidate (dopamine reuptake inhibitor) might also be used to substitute for intravenous amphetamine use. On the basis of this information, we aimed to compare the effectiveness of aripiprazole, methylphenidate, and placebo in the treatment of amphetamine dependence using urinalysis as an objective measure of primary outcome.
Method
It was estimated that a group size of 70 individuals was sufficient to detect an effect of medium magnitude (alpha=0.05, beta=0.80). Therefore, we aimed to randomly assign 70 subjects to each group, resulting in a total number of 210 subjects. However, after obtaining unexpected results from interim analysis of the first 53 patients (one active medication arm being significantly worse than placebo arm) and contacting the local ethical committee and the National Agency of Medicines, the enrollment was discontinued.
All study subjects gave written informed consent. The inclusion criteria were amphetamine (or methamphetamine) dependence (per DSM-IV), age between 18 and 65, recent and accustomed intravenous amphetamine/methamphetamine use, confirmed by urinalysis. The study protocol was approved by the local ethical committee. Details of the subject progression through the trial are included in the supplement that accompanies the online version of this article. The study was registered by Current Controlled Trials Ltd (http://www.controlled-trials.com).
The eligibility of the potential study subjects was assessed during a 2-week screening period, after which subjects included in the study started to receive oral regimens of aripiprazole (15 mg/day), methylphenidate (18 mg/day for the first week, 36 mg/day for the second week, and 54 mg/day thereafter) or placebo in identical gelatin capsules for 20 weeks. The medication was given daily under staff supervision (the patient had to swallow the capsule and then drink a sufficient amount of water). The urine samples were obtained twice a week under supervision. All patients received unstructured psychosocial treatment with elements of cognitive therapy and psycho-education, counseling, and support.
The primary outcome measure was the proportion of amphetamine-positive urine samples during pharmacological treatment. An intention-to-treat analysis was used, and all missing samples were considered to be amphetamine positive. The data were analyzed by a logistic regression model. Change in amphetamine use (proportion of positive urine samples) as a function of time in each treatment group was tested with cumulative sums method. Cumulative sums method tests existence of any change as a function of time in proportion of positive urine samples, and if change is observed, detects the change point (6). The retention in treatment was analyzed with Cox’s proportional hazard regression analysis. All data analyses were carried out using R software (7).
Results
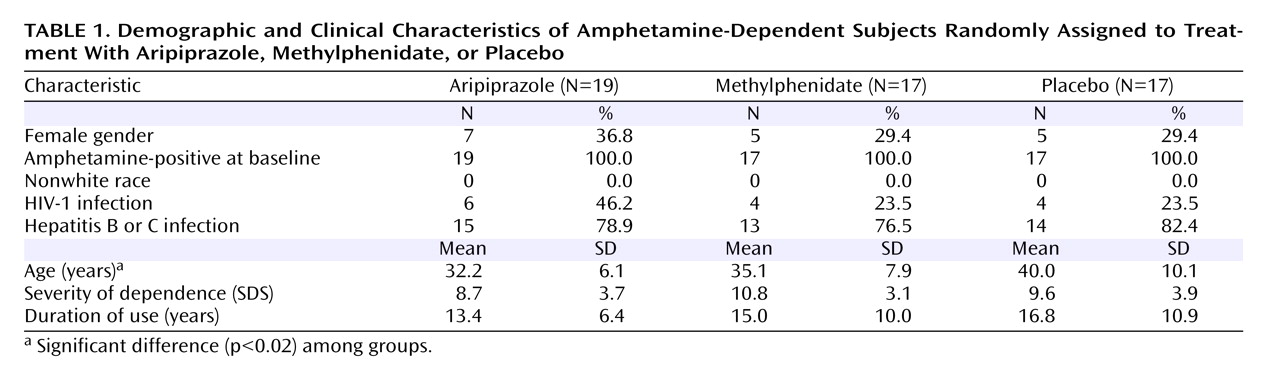
The study groups differed concerning their mean age, but not in baseline severity of dependence or other clinical or demographic characteristics (
Table 1 ). None of the subjects were found to be using methamphetamine. No significant difference among the three treatment arms was observed in study retention (p=0.32, age-adjusted p=0.47). Fourteen patients gave at least one amphetamine-negative urine sample. No significant differences were observed in the baseline characteristics between these patients versus those 39 patients who gave no amphetamine-negative samples. The mean proportion of amphetamine-positive urine samples was 90.7% in the aripiprazole group, 67.3% in the methylphenidate group, and 82.0% in the placebo group in observed cases (missing samples ignored). These values were 99.1% (aripiprazole), 93.5% (methylphenidate), and 97.2% (placebo) when all missing urine samples (considered as positive) were included in the intention-to-treat analysis (number of samples for each patient: 40). During the second half of the follow-up period, i.e., during the last 10 weeks, the mean proportion of amphetamine-positive urine samples was 100% in the aripiprazole arm, 46.3% in the methylphenidate arm, and 79.1% in the placebo arm in the observed samples analysis, and 100% in the aripiprazole arm, 91.5% in the methylphenidate arm, and 97.4% in the placebo arm in the intention-to-treat analysis.
Two patients in the aripiprazole group were removed from the trial because of a worsening in their physical condition: one patient developed a transient increase of liver enzymes, attributed to recently started HIV medication, and the other incident was a transient ischemic attack that was attributed to continued amphetamine use. All other recorded adverse events were mild and did not lead to premature discontinuation of the medication. The frequency of reported side effects did not differ between the treatment groups.
In the intention-to-treat analysis, patients allocated to aripiprazole had a higher proportion of amphetamine-positive urine samples than patients who had received placebo (odds ratio=3.09, 95% CI=1.29–7.40; z=2.53, p=0.01). The patients allocated to methylphenidate had fewer positive urine samples than patients in the placebo group (odds ratio=0.42, 95% CI=0.24–0.72; z=–3.14, p=0.002). Since the treatment groups differed in mean age, the age-adjusted odds ratios were also calculated. Adjusted odds ratios were 3.77 (95% CI=1.55–9.18; z=2.92, p=0.003) for aripiprazole and 0.46 (95% CI=0.26–0.81; z=–2.67, p=0.008) for methylphenidate. The fluctuation in the proportion of amphetamine-positive samples (only the analyzed samples included) showed that the significant reduction in amphetamine use was observed after 18 weeks in the methylphenidate arm (p value for change from the baseline was 0.01 for methylphenidate, 0.71 for aripiprazole, and 0.79 for placebo).
Discussion
Our results indicate that methylphenidate treatment is associated with a statistically significant reduction in intravenous amphetamine use when compared with placebo, providing the first controlled evidence of an effective pharmacological treatment for amphetamine dependence. On the contrary, aripiprazole treatment was associated with a higher proportion of amphetamine-positive urine samples than placebo.
While aripiprazole (in amphetamine dependence) and naltrexone (in opioid dependence) may be good treatments in theory, it seems that effective pharmacological maintenance treatments for intravenous drug dependence are substances that induce at least some euphoria, such as methadone and buprenorphine in opioid dependence (3), or methylphenidate in amphetamine dependence. Slow-release methylphenidate may be superior to usual short-acting formulation, since the patient may start experiencing cravings for amphetamine as soon as the effect of the substitute drug disappears. It is likely that methylphenidate should be dispensed mostly on a daily basis under supervision because of its abuse potential. Aripiprazole (15 mg/day) was not effective in this trial (an abstinence facilitation trial), but we cannot draw any conclusions on its potential efficacy in a relapse prevention study among detoxified patients.
These results show that amphetamine use began to decrease substantially as a function of time after 10 weeks of methylphenidate treatment reaching statistical significance at 18 weeks, which indicates that it may take an even longer period of time than 20 weeks to achieve full benefit from this treatment. The exclusion criteria in the present study were mainly due to safety issues, and the patient population included in the study, having a severe dependence and long history of intravenous drug use, was practically similar to that found in real-life clinical practice. Therefore, this trial was more of an effectiveness study, rather than an efficacy study, and these results can be generalized to real-life settings.
Urinalysis may underestimate the decrease in drug intake, since decline in drug use from several times a day to once a day would indicate no change (8). In this study, the difference between methylphenidate and placebo in the proportions of amphetamine-positive urine samples was 6 percentage units in the intention-to-treat analysis and 33 percentage units in observed samples analysis during the second half of the follow-up phase. It is likely that a large proportion of those patients who were removed from the trial because they stopped visiting the clinic for a time period longer than 7 days would continue to be treated in usual practice. Thus, it can be estimated that absolute risk reduction attributable to methylphenidate treatment measured with urinalysis in a real-life setting might be between 6 and 33 percentage units, and that the figures for the decrease in actual drug use would be probably even greater. An effect of this magnitude should result in a significant reduction of the collateral medical and social problems related to intravenous amphetamine use.