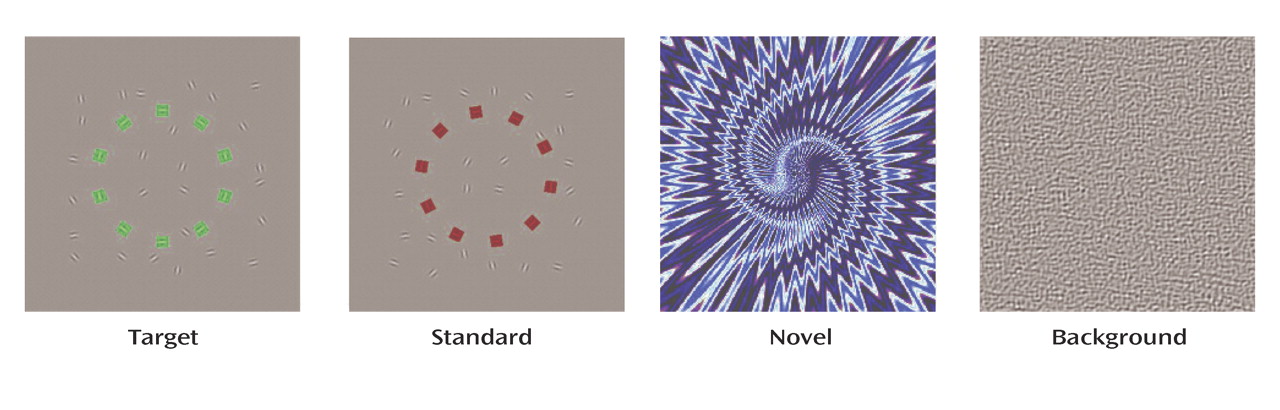
We applied the visual oddball paradigm with event-related fMRI to examine regional hemodynamic responses to targets and novel distractors. We hypothesized that the abnormalities in brain activity responsible for reduced P3b and P3a event-related potential responses in schizophrenia would be reflected by abnormal hemodynamic activations for targets and for distractors, respectively. Reduced event-related potential amplitudes could result from attenuated activation in regions generating P300 subcomponents. Alternatively, there could be changes in regions that do not actually generate P300 but serve as modulators that can either facilitate or disrupt neuronal synchrony of underlying generators. Therefore, we examined regions of abnormally decreased and increased activation in patients. Finally, activation abnormalities were related to the patients’ performance and symptoms.
Discussion
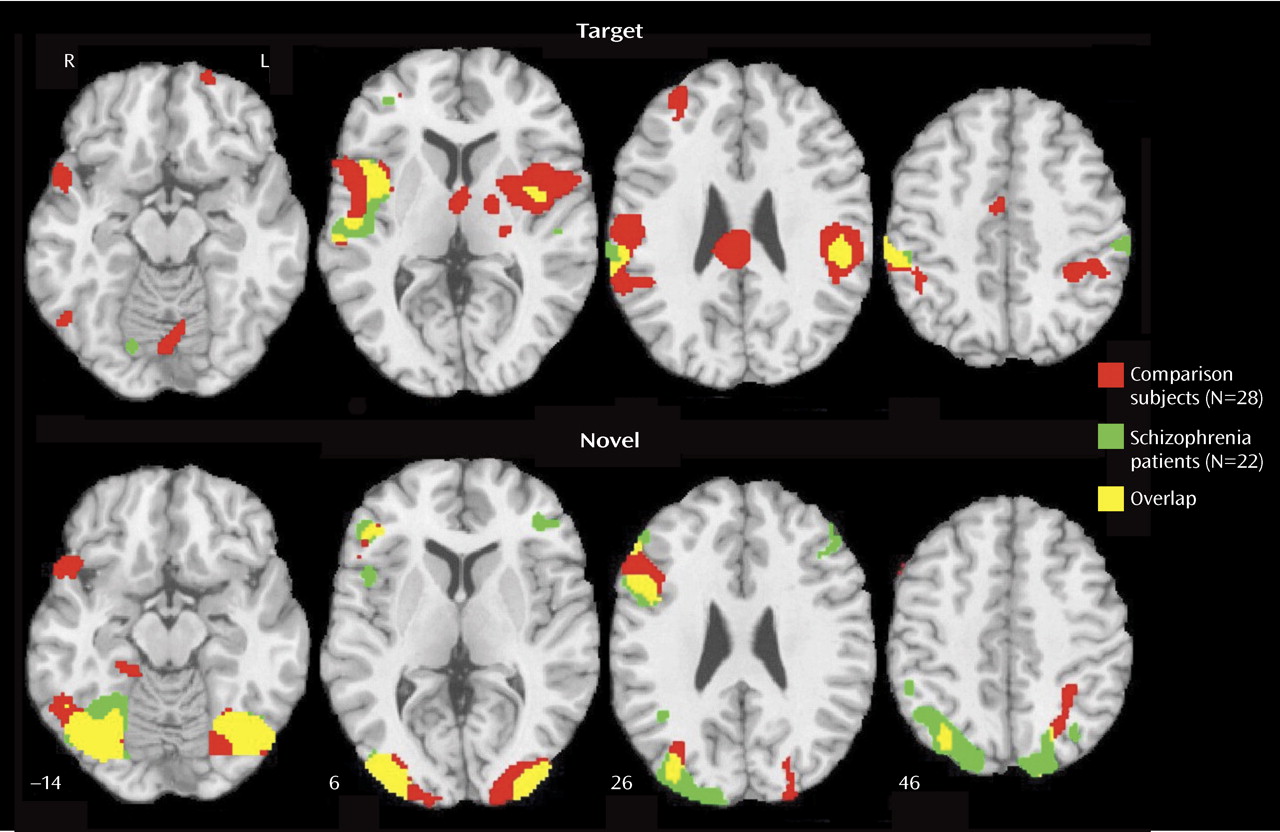
Patients with schizophrenia and healthy participants showed robust regional cerebral activation temporally associated with the appearance of targets. Consistent with fMRI studies of spatial attention
(22,
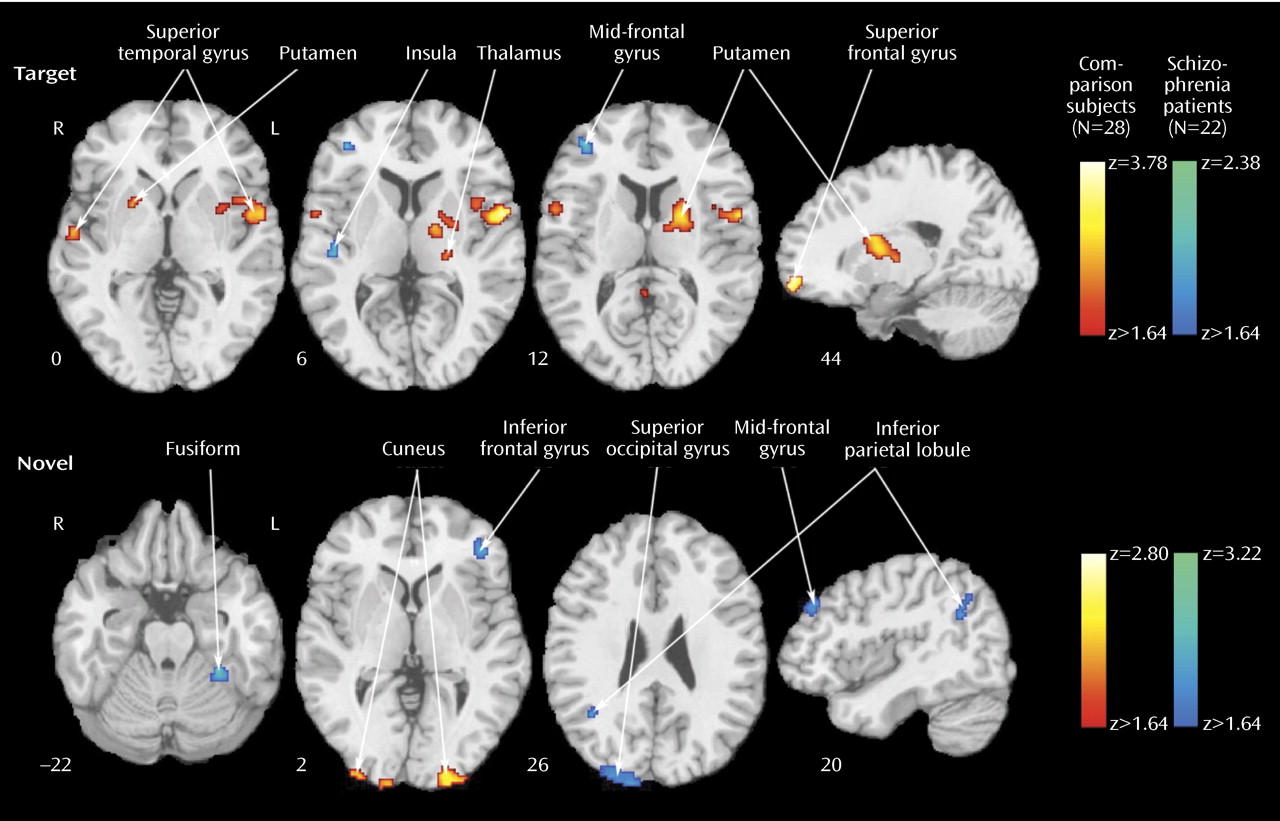
23), both groups recruited temporal, parietal, cingulate, and frontal regions. Despite equal performance, the patients activated some but not all regions activated by comparison subjects. They had diminished activation in the superior temporal gyrus bilaterally, the thalamus and basal ganglia, and superior frontal areas. These regions are important in sensory input integration and response selection
(24) and have been implicated in schizophrenia
(5,
11) . Because the task was made easy to match the performance of patients and comparison subjects, different activation patterns likely reflect the use of alternative strategies. Failure to activate brain circuitry recruited by healthy people would be expected with dysfunction in that circuitry. Such failure of activation may generate performance deficits in more demanding attentional tasks.
In few regions, specifically the left inferior parietal lobule, the right insula, and the mid-frontal gyrus, the patients showed increased activation for targets relative to comparison subjects. The patients also activated a right posterior cingulate region not activated in comparison subjects. Both fMRI
(25,
26) and focal lesion studies
(27) have linked the inferior parietal lobule to the auditory target P300 response. Increased activation in schizophrenia of the left inferior parietal lobule and the right mid-frontal gyrus has been reported for working memory
(28), suggesting overexertion. Activation of the right insula has been related to risk-taking during decisions
(29), while posterior cingulate activation has been linked to thought suppression and cognitive control
(30) .
The results suggest that attentional processing in schizophrenia is compromised by increased demands on working memory to complete even simple tasks, combined with greater risk assignment to decisions and the increased need to suppress competing thoughts. Consistent with reports on neural circuitry involved in response to novel visual stimuli
(6,
7,
31), a more posterior brain system responded to their presentation. Activation was prominent in occipital-temporal regions, with only right inferior frontal gyrus activated anteriorally. In contrast to targets, for which patients activated fewer regions and less robustly, for novels, patients activated almost all regions activated in comparison subjects and some additional areas.
Furthermore, most between-group differences indicated greater activation in patients. The areas of reduced activation in patients included the visual cortex and the left inferior parietal lobule, which were overactivated in patients as targets. The reduced bilateral activation in the cuneus suggests less mobilization of visual association areas, whereas reduced activation in the inferior parietal lobule suggests less allocation of attentional resources. Increased activity in patients for novel distractors was observed in occipital-parietal regions, reflecting increased activity superiorly. Greater occipital activation in dorsal regions and in the left fusiform gyrus may reflect efforts in visual processing of the spatial layout
(32) . Notably, the patients activated two frontal regions, the right mid-frontal gyrus and the left inferior parietal lobule, not activated in comparison subjects. This likely reflects increased efforts by the patients to inhibit responses to distractors compared to the normal subjects, who had inhibited the response to distractors at earlier stages of processing in the posterior visual system
(11) .
Several regions were activated by both targets and distractors: the right superior temporal gyrus and mid-occipital gyrus and the left inferior frontal gyrus and lingual gyrus. These areas likely mediate visuospatial integration and attentional requirements to both conditions. Because the inferior parietal lobule is activated by both targets and distractors, reduced activation for distractors in patients, in the context of increased inferior parietal lobule response to targets, may reflect limited resources for serving the conflicting needs of regulating top-down requirements and bottom-up interference. When we consider the pattern of abnormal activation and failure to activate in schizophrenia, it appears that attentional deficits emanate from both insufficient recruitment of regions necessary for goal-directed behavior and overprocessing of irrelevant events.
The correlations between regional activation and response time provide further validation that activation measured by the BOLD signal reflects the operation of a neural system that facilitates attentional processing. In comparison subjects, the main regions activated for target processing, including the bilateral superior temporal gyrus, left inferior parietal lobule, thalamus, cingulate, and right mid-frontal, also show response facilitation with increased activity. Such correlations indicate that activation of this cortical-thalamic network facilitates response to targets in attentional settings. The attenuation of these correlations in patients, despite comparable performance, suggests the operation of other factors that may include medication effects.
The correlations between hemodynamic response to distractors and processing efficiency for targets suggest a linkage. In comparison subjects, activation to distractors predicting faster response time for targets occurred in regions activated by targets. The only exception was the precuneus, which was activated by novels and predicted faster response to targets. This indicates a network that distinguishes salient events that require a response from distractors and supports the contribution of the precuneus in processing novel visual stimuli for top-down relevance
(33) . In patients, anterior cingulate and superior occipital activation to distractors accelerated response to the target, whereas activation of the left superior temporal gyrus slowed responses. This supports left superior temporal gyrus involvement in schizophrenia
(5) .
The correlations between activation measures and symptom severity also revealed regional specificity. Greater activation of right temporal regions was associated with milder negative symptoms, whereas greater bilateral frontal activation was associated with increased symptom severity.
The correlations for temporal regions are consistent with group differences in activation. Because patients had reduced temporal activation, normalizing activation predicted less severe symptoms. For frontal regions, activation was associated with more negative symptoms, although patients overactivated the right mid-frontal and underactivated the left superior frontal areas. This suggests that negative symptoms are associated with a greater need to recruit frontal regions for target detection. For distractors, correlations with SANS were also negative for temporal and positive for frontal activation. For SAPS, target and distractor activation of frontal regions was associated with fewer symptoms, as was activation of the anterior cingulate for targets and the basal ganglia and precuneus for distractors. Thus, frontal activation portends less severe positive and increased negative symptom profiles. Perhaps recruitment of frontal regions in target detection indicates executive difficulties associated with negative symptoms, whereas recruitment of frontal regions in response to distractors indicates excessive processing of irrelevant information, mediating positive symptoms.
Although parallel measurements of electrophysiology and fMRI are needed to assess such relationships directly, our results suggest potential metabolic substrates for the visual P300 and its abnormalities in schizophrenia. Visual P3a and P3b likely have separable but partially overlapping generators, with the inferior parietal lobule, superior temporal gyrus, and visual association areas providing a system that responds to stimulus deviance regardless of whether it is a target or a novel distractor. These regions have been implicated in both auditory and visual P300 electrophysiological studies. Likewise, the regions activated specifically for targets and novels have been related to P3b and P3a generation, respectively
(26 –
28) . Reduced P3a and P3b in schizophrenia, however, may be caused by different mechanisms. Reduced P3b seems to result from failure to recruit regions that generate it because target response in patients was less robust. By contrast, reduced P3a magnitude in schizophrenia may relate to overactivation of regions that are not engaged by healthy people, attenuating its propagation. Notably, the left inferior parietal lobule, putatively involved in generating both P3a and P3b, was overactivated in patients for targets but underactivated for distractors.
The present study has several limitations. The patients were stable, mildly symptomatic, and medicated. Our positive results encourage more systematic evaluation of such factors, including animal studies to clarify medication effects on response to targets and novels. We used the visual modality, more amenable to fMRI. Most electrophysiological studies have examined the auditory modality, which may have more extensive abnormalities in schizophrenia. The generalizability of our findings to other visual targets and novel stimuli also needs further investigation. For example, to ensure that the novel stimuli are “attention grabbers,” we have made them complex. Although we have equated targets and novels for size and luminance, this dimension requires further examination by manipulating the relative complexity of targets and distractors. For example, familiar distractors can be used that are of equal complexity to targets.
Notwithstanding these limitations, the results demonstrate robust effects of target detection and novelty processing in patients and comparison subjects but several noteworthy differences. Our study simulates a situation in which an individual is asked to pay attention to a specific infrequent event while being intermittently distracted by attention-grabbing but irrelevant events. We observed that patients showed diminished activation for the targets in key regions, yet for novel distractors, they overactivated several regions. Appropriate activation of networks regulating target and novelty processing was generally associated with faster performance and, in patients, with less severe symptoms. These results encourage further efforts to examine attentive and preattentive processing in schizophrenia with event-related fMRI.