Excessive sensitivity to negative environmental cues has long been posited to play a significant role in the etiology and maintenance of major depressive disorder
(1,
2) . In the clinic and laboratory, depressed individuals consistently magnify the significance of failure
(3,
4), exhibit bias toward negative (or against positive) self-descriptors
(5), have difficulty recovering once an error has been committed, and show detrimental sensitivity to mistakes and negative feedback
(6 –
8) . Such impairments are consistent with cognitive theories of depression that suggest emotional and behavioral manifestations of depression are maintained by an automatic tendency to distort environmental information in a negatively biased way
(1,
2) .
In major depression, this enhanced sensitivity to negative environmental cues appears to be coupled with decreased hedonic capacity as well as reduced responsivity to pleasant stimuli and other positive reinforcers, such as monetary incentives or favorable social cues
(9 –
12) . In addition, dysphoric participants consistently exhibit equivalent behavioral responses to neutral and rewarding payoff conditions, whereas nondepressed individuals develop response biases toward rewarding stimuli
(13,
14) . Functional neuroimaging investigations, pharmacologic challenge studies, and animal models of depression provide converging evidence that reward-related neural pathways are hyporesponsive to rewarding stimuli yet show hyperresponsivity to dopamine agonists in depressed individuals
(15 –
17) .
A growing literature suggests that increased activity in paralimbic emotion-related neural structures and aberrant connectivity among these regions contribute to maladaptive affective reactions and sensitivity to negative cues in major depression
(18 –
21) . That is, depressed individuals appear to exhibit an exaggerated response in the anterior cingulate cortex that contributes to an imbalance in corticolimbic networks that shifts influence to limbic regions. Indeed, researchers have observed increased metabolism in the rostral anterior cingulate cortex coupled with decreased activity in prefrontal cortical areas among nondepressed participants in whom sadness is induced
(20) . Similarly, depressed individuals show increased activation of the anterior cingulate cortex and paralimbic brain structures in response to negative stimuli
(18) . Metabolic studies further suggest that depressed individuals show high baseline cerebral blood flow in rostral anterior cingulate regions
(19) . Together, these data indicate that excessive sensitivity to negative cues may be associated with enhanced activity in regions of the anterior cingulate cortex and anomalous corticolimbic connectivity, which may then contribute to the disruption of emotion regulation processes.
Although these neural data and concomitant behavioral and affective symptoms are central to the clinical presentation and current conceptualizations of major depressive disorder, much remains unknown regarding the mechanisms that contribute to the maladaptive responses to negative stimuli. The temporal and functional resolution of event-related brain potentials allows the investigation of specific neural and cognitive processes associated with the hypersensitivity to negative cues seen in major depression. In the present work, the error-related negativity and error positivity components of the event-related potential facilitated the assessment of sequential error-processing mechanisms in depressed patients.
The error-related negativity is an event-related potential component that manifests 50–100 msec after incorrect responses in a variety of binary choice tasks
(22,
23) . The component is reliably measured at midline frontocentral scalp sites and is considered to be a robust electrophysiological measure of the initial engagement of an intact response-monitoring system that provides resources for early detection of an error or conflict
(23,
24) . Studies using source localization and functional magnetic resonance imaging (fMRI) converge on regions of the anterior cingulate cortex as probable neural generators of the error-related negativity component and related error processing
(25 –
27) . Given the substantial evidence that individuals with depression show excessive sensitivity to errors, failure, and negative cues along with evidence of enhanced activity in error-related regions of the anterior cingulate cortex
(2 –
4,
21), a primary goal of the present work was to test the hypothesis that depression is characterized by hypersensitivity to errors in early stages of error detection, as would be indicated by enhanced amplitude of the error-related negativity component. To date, we know of no published work that has examined error-related negativity in individuals in a major depressive episode relative to nonpsychiatric comparison subjects (see reference
28 for a preliminary study on geriatric individuals with current and remitted depression).
An error-locked centroparietal positivity
(29 –
31) occurs subsequent to the error-related negativity, typically within 200–400 msec after incorrect responses. Increasing evidence indicates that the error positivity component reflects recognition of an error, as indicated by the presence of smaller error positivity (but unchanged error-related negativity) in response to unperceived errors than in response to perceived errors
(30) . As targeted investigations of error positivity are scarce and have not, to our knowledge, been thus far examined in major depression, the present study did so as an exploratory aim.
As already outlined, depressed individuals exhibit heightened sensitivity to error-related information and negative environmental cues, along with reduced responsivity to positive reinforcers. In order to clarify the neural processes contributing to such symptoms and assess the sensitivity of these measures to motivating task incentives, event-related potentials associated with sequential aspects of error processing were measured as participants with and without major depressive disorder performed a common speeded response-competition task that reliably elicits errors and their accompanying physiological processes
(22) . Physiological and behavioral responses to errors in this task were measured in a neutral condition and under positive and negative task contingencies.
Method
Participants
The participants were 18 individuals with major depressive disorder who were experiencing a current major depressive episode and 17 nonpsychiatric comparison subjects. All of the volunteers were recruited from community and mental health centers throughout the Greater Boston area through newspaper, Internet, and poster advertisements. Following an initial telephone screening in which basic exclusion criteria were applied, qualifying participants were invited to the laboratory for diagnostic assessment. The basic exclusion criteria included left-handedness, history of seizures or stroke, head injuries resulting in more than 10 minutes of unconsciousness or with neurological sequelae, hormone disorder, history of ECT or chemotherapy for cancer, current pregnancy or menopause, and history of substance abuse or dependence. In addition, the exclusion criteria for the comparison subjects included any current or past axis I diagnosis.
Psychiatric inclusion and exclusion criteria were assessed with the Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Patient Edition (SCID-I/P). All diagnoses were based on the consensus of two independent trained doctorate-level raters (P.C. and another rater). Written informed consent was obtained during the first laboratory visit, after a complete description of the study and procedures to the participants.
Among the depressed individuals, the average length of the current depressive episode was 22 months, and the average number of prior episodes was 2.5. Five depressed participants also received comorbid diagnoses of the following anxiety disorders: social phobia (N=1), panic disorder (N=1), panic disorder and social phobia (N=2), and posttraumatic stress disorder (N=1). Six individuals were taking antidepressant medication at the time of the study; five of these six were concurrently undergoing psychotherapy. Analyses of the physiological measures did not yield statistically significant differences between individuals with and without treatment; thus, all individuals were included in the reported analyses. (It should be noted, however, that the low numbers of subjects—six medicated and 12 unmedicated depressed participants—may not yield sufficient statistical power to detect true between-group differences. Thus, the possibility that antidepressant medications affect the amplitude of error-related negativity or error positivity remains to be examined in future investigations.)
The depressed and comparison groups did not differ significantly in age (comparison: mean=37.9 years, SD=13.1; depressed: mean=33.7 years, SD=12.5) or years of education (comparison: mean=16.1 years, SD=2.0; depressed: mean=15.7 years, SD=2.2) (two-tailed t tests, p>0.30 for both). The two groups also had similar proportions of Caucasian participants (comparison: N=14; depressed: N=15) and men (comparison: N=10; depressed: N=6) (chi-square analyses, p>0.10 for both).
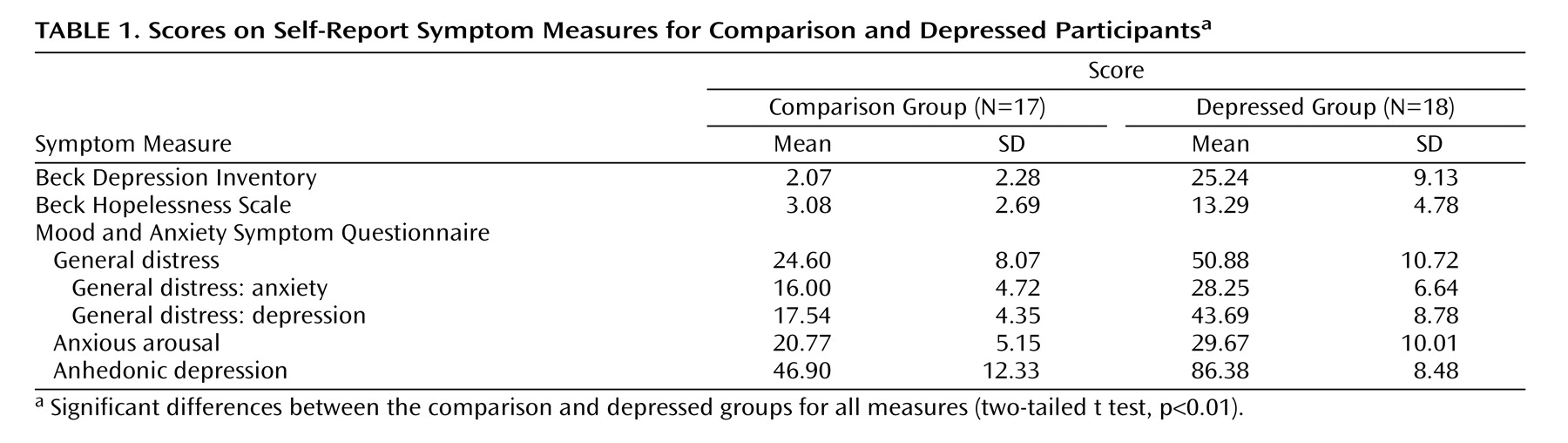
Self-Report Measures
Prior to physiological recording, the participants completed the Beck Depression Inventory (BDI), the Beck Hopelessness Scale
(32), and the Mood and Anxiety Symptom Questionnaire
(33) . These were chosen to measure the current severity of depression symptoms, hopelessness, and the presence of depression relative to anxiety symptoms, respectively. The two groups differed predictably on all measures, and the scores are presented in
Table 1 .
Task
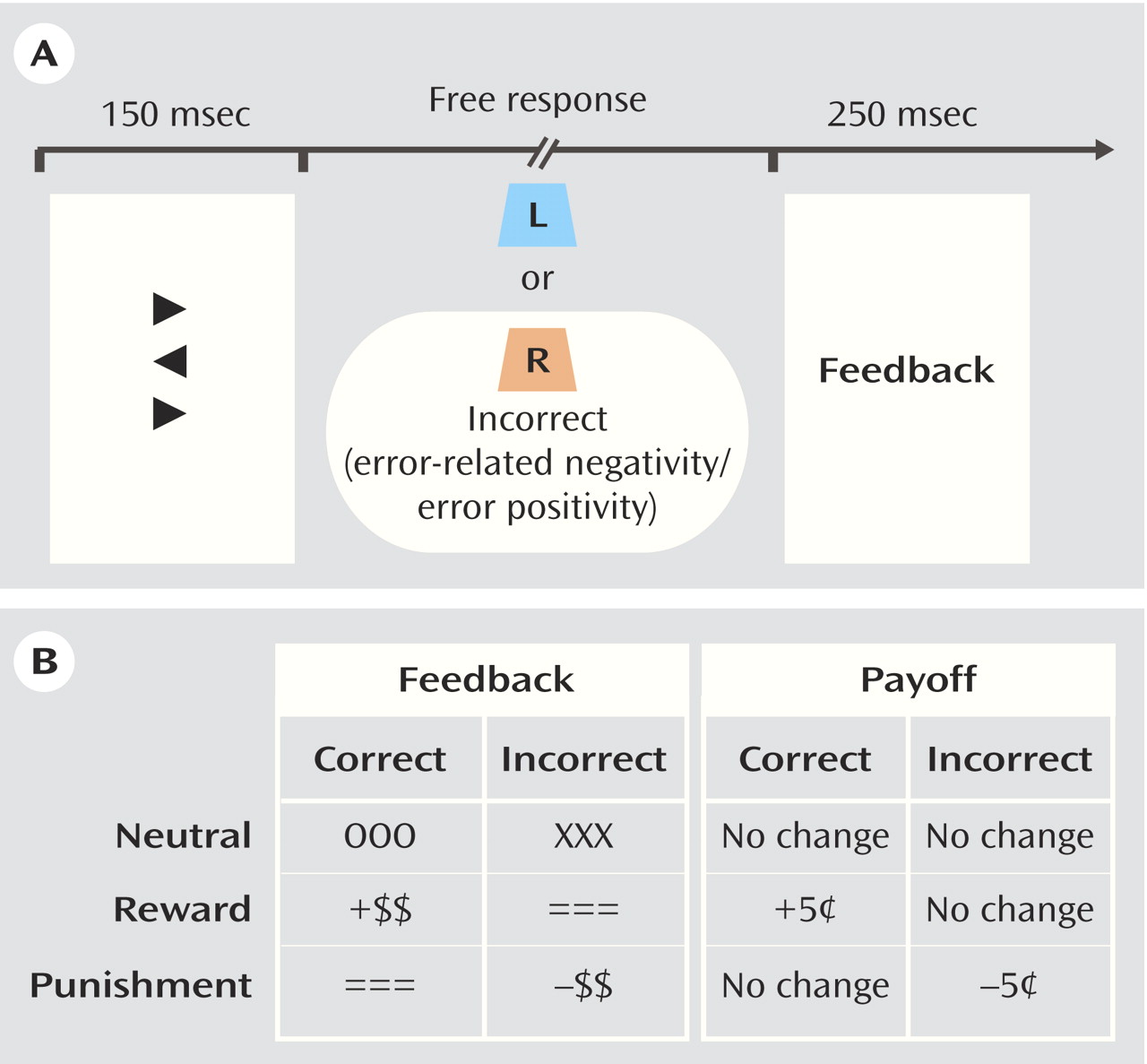
The primary task was a modified flanker task in which a target arrow is flanked on the top and bottom by congruent, incongruent, or neutral distractors
(34) . Participants were given a two-button response box and instructed to respond in the direction of the target arrow. Accuracy feedback, varying by incentive condition, was presented for 250 msec at 1300 msec following the response in each trial. A variable intertrial interval (range=1500–2500 msec), measured from feedback offset to onset of the subsequent flanker stimulus, was used. See
Figure 1 for a schematic representation of the procedure.
The participants performed 360 trials in each of three conditions, presented in a block design. Each physiology recording session began with the “neutral” condition, followed by two counterbalanced “incentive” conditions. In each incentive condition, the participants were informed that they could earn up to an extra five dollars. Specifically, in the “reward” block, the participants were told they would receive “an extra five cents for each correct response, and nothing for incorrect responses.” In the “punishment” block, the participants were informed they would begin with the full amount and would “lose five cents for each incorrect response.” Accuracy and speed were equally emphasized, and in order to maintain orientation to the task, a feedback message of “late” was presented regardless of accuracy when a participant’s reaction time exceeded a timeout criterion, which was calculated as 85% of the individual’s mean reaction time
(23) during a practice block preceding the physiology recording. The practice consisted of 60 trials, during which the flanker task was performed without visual feedback; the participants were informed that this was a practice block and were simply instructed to respond as quickly and accurately as possible. The comparison and depressed groups did not differ in reaction time or accuracy in the practice block.
Physiological Recording and Data Reduction
EEG was recorded from 25 scalp sites by using a custom 37/64 channel bioelectric amplifier (S.A. Instrumentation, Encinitas, Calif.) and a stretchable cap of spandex-type fabric (Electro-Cap International, Eaton, Ohio) with Ag/AgCl electrodes positioned according to the International 10–20 system. The data were digitally sampled at 1000 Hz and analog filtered between 0.01 and 100 Hz. Electrode impedances were kept below 5 kΩ. Electro-oculogram (EOG) data were recorded from electrodes placed lateral to the outer canthi and at the left supraorbital and suborbital positions. EEG was referenced to the left mastoid (M1) and algebraically re-referenced to average mastoids off-line ([M1+M2]/2). EOG artifact was corrected by using the regression method of Gratton et al.
(35) implemented by BrainVision Analyzer software (Brain Products, Gilching, Germany). Individual trials exceeding 80 μV were automatically rejected; remaining EOG and electromyography artifacts were manually identified and rejected from further analyses.
Statistical Analyses
The response-locked individual event-related potentials were averaged for each error trial in the neutral and incentive (reward, punishment) conditions. Error-related negativity was calculated as the most negative peak in a window –30 to 150 msec around the response, relative to a –100 msec baseline, and error positivity was quantified as the most positive peak following the error-related negativity in a window 100–400 msec from response onset.
An initial 5×5×2 multivariate analysis of variance (MANOVA) with Wilks’s lambda correction was performed for the peak amplitudes for error-related negativity and error positivity in the neutral condition in order to identify regions of maximal activation and between-group differences in component amplitudes. The three variables included in the MANOVA were caudality (frontal, frontocentral, central, centroparietal, parietal), laterality (electrode sites 7, 3, z, 4, 8), and group (comparison, depressed). The additional factor of incentive (reward, punishment) was included in this omnibus MANOVA in analyses of the incentive conditions. Subsequent incentive-by-group MANOVAs were implemented at regions of maximal activation, with the factors of caudality and/or laterality included as indicated from the larger MANOVA. Interactions were required to be significant at each stage before further decomposition, and significant main effects were followed with pairwise comparisons as appropriate. All statistically significant effects from these analyses are reported in the Results section.
In order to assess within- and between-group behavioral differences, univariate repeated-measures analyses of variance (ANOVAs) assessing the interaction of group (comparison, depressed) and accuracy (correct, incorrect) were performed for response latency, response accuracy, post-error latency, and post-error accuracy in the neutral feedback condition. In addition, group-by-accuracy-by-incentive ANOVAs were performed for each of these measures in the incentive conditions. Finally, Pearson correlations were used to examine the relationship of the symptom and behavioral variables to the amplitudes of error-related negativity and error positivity at regions of maximal activation in the neutral condition.
Results
Behavioral Outcomes
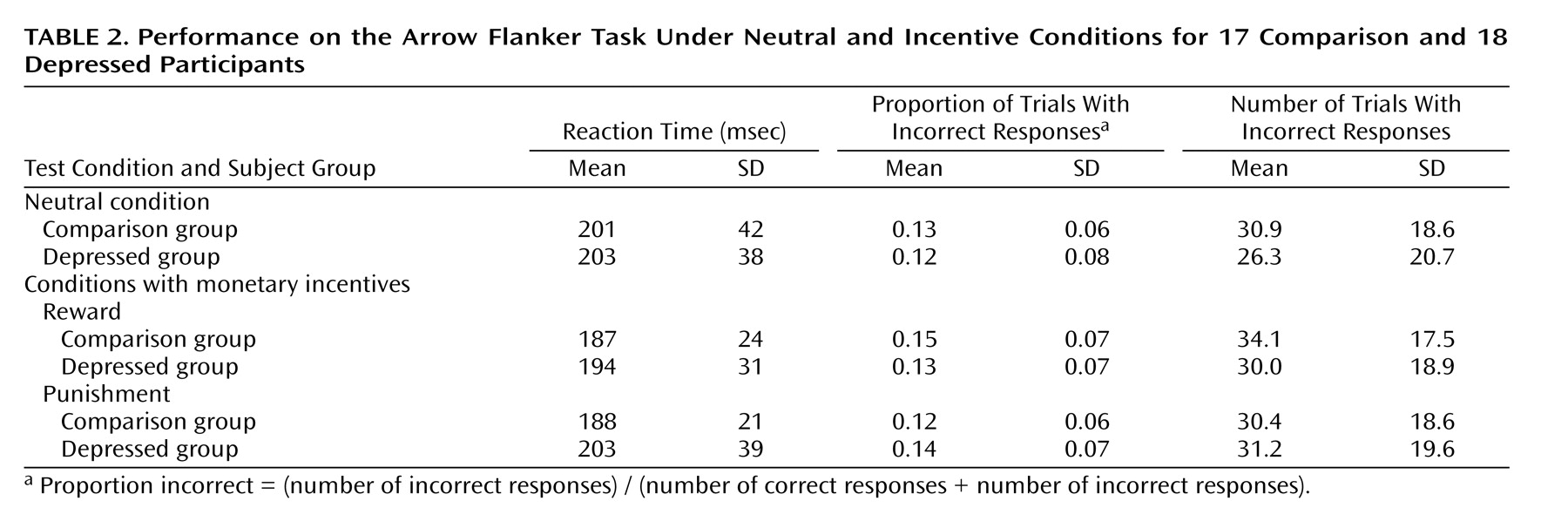
Neutral condition
In the neutral condition, the comparison and depressed groups performed equally accurately (main effect of group: F=2.1, df=1, 33, p>0.10). The effects of post-error accuracy adjustments were intact such that all participants were more accurate after incorrect trials than after correct trials (main effect of accuracy: F=23.2, df=1, 33, p<0.001). Moreover, the depressed group showed greater post-error increases in accuracy than did the comparison group (group effect: F=4.2, df=1, 33, p<0.05).
The comparison and depressed participants exhibited equivalent reaction times. The response latencies for incorrect trials were shorter than those for correct trials (accuracy effect: F=199.5, df=1, 33, p<0.001). No effects of the post-error adjustment on response latency were observed (accuracy-by-group interaction: F=0.0, df=1, 33, n.s.).
Incentive conditions
The depressed and comparison groups were equally accurate across the punishment and reward conditions (group effect: F=2.8, df=1, 33, p>0.10). Post-error accuracy adjustment (greater accuracy after incorrect trials than after correct trials) was intact under both incentive conditions across groups (accuracy effect: F=39.4, df=1, 33, p<0.001). No interactions or main effects involving the group factor were observed for accuracy in the incentive conditions.
Across incentive conditions, response latencies for incorrect trials were again shorter than those for correct trials (accuracy effect: F=6.2, df=1, 33, p<0.05). Moreover, response latencies in the reward condition were significantly shorter than in the punishment condition for all participants (main effect of incentive: F=440.1, df=1, 33, p<0.001). The effect of the post-error adjustment on response latency was intact such that all participants were slower after error trials than after correct trials (accuracy effect: F=394.2, df=1, 33, p<0.001). No interactions or main effects involving the group factor were observed for response latencies in the incentive conditions.
The mean response times, error rates, and number of trials comprising each condition are presented in
Table 2 .
Error-Related Negativity
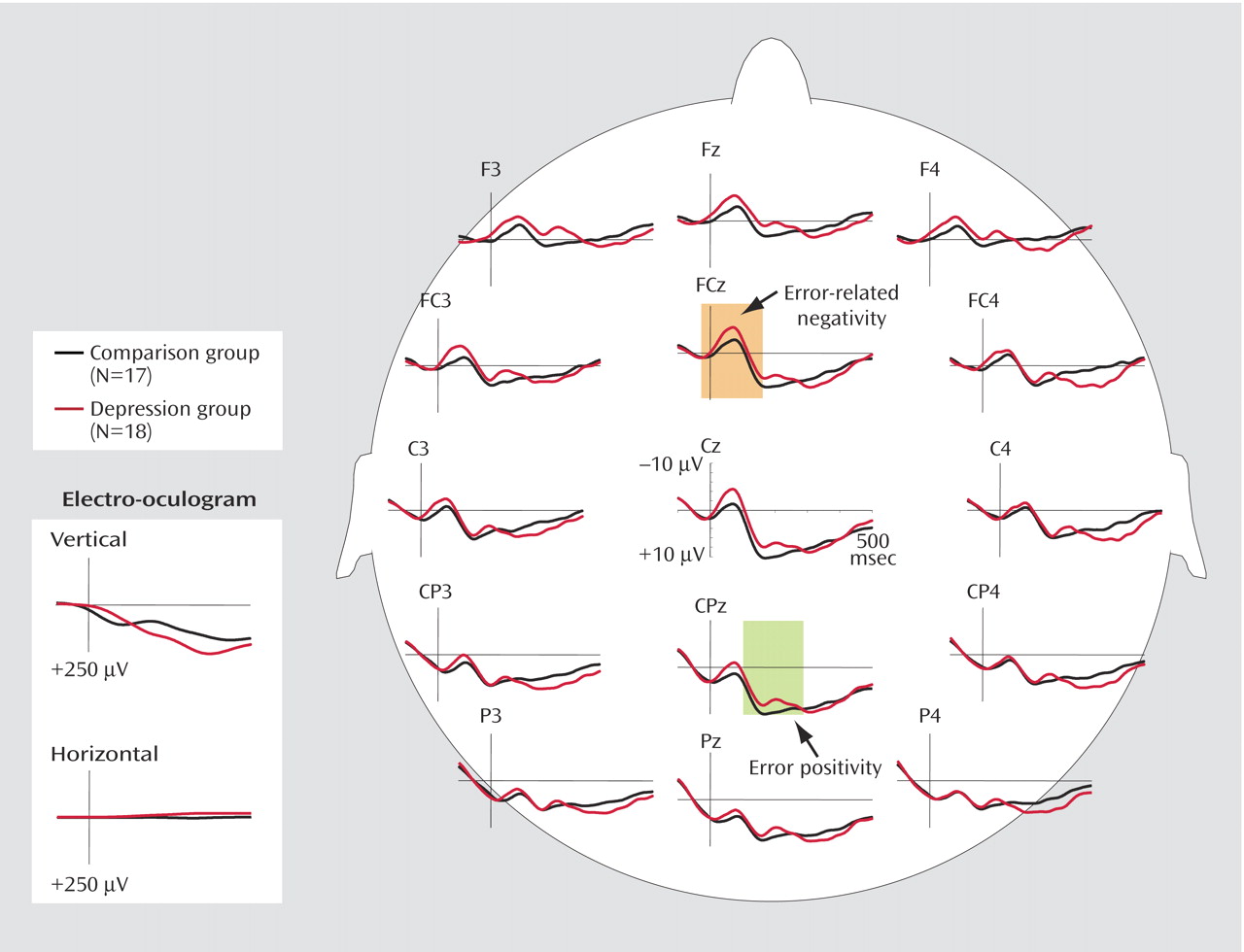
Neutral condition
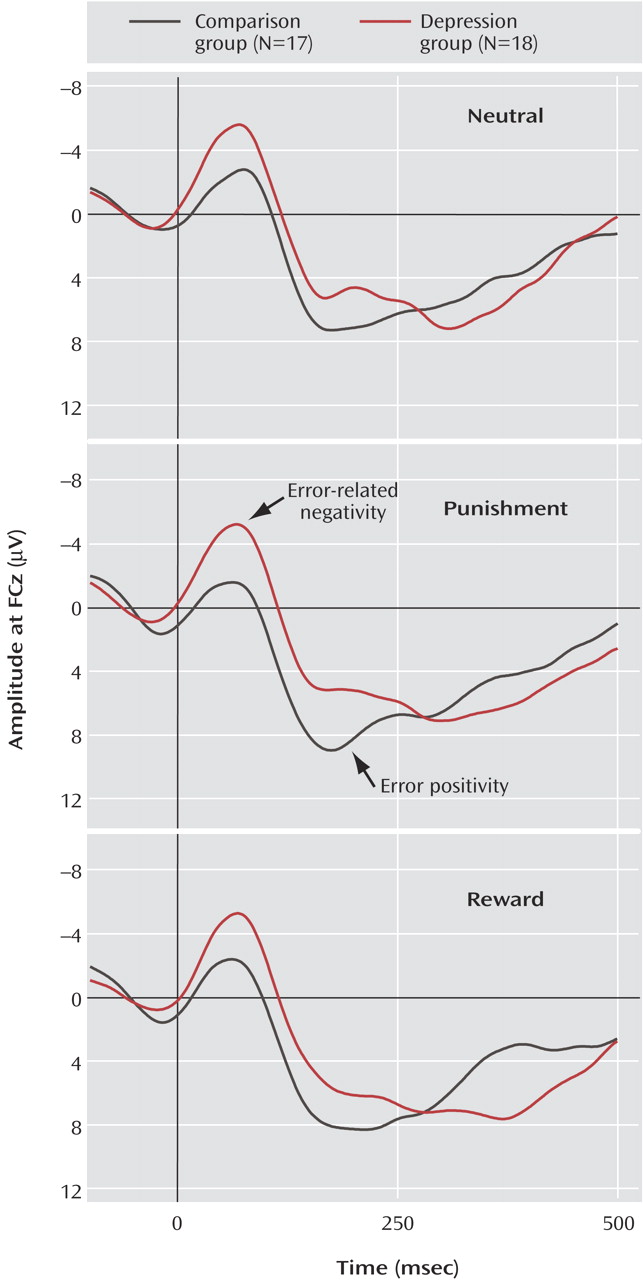
Figure 2 depicts grand average waveforms at midline and lateral sites for both groups. The amplitudes of error-related negativity for error trials showed significant main effects of both caudality and laterality (caudality effect: F=17.1, df=4, 27, p<0.001; laterality effect: F=19.3, df=4, 27, p<0.001). Subsequent pairwise comparisons revealed that for all participants, error-related negativity was greatest at the frontal and frontocentral midline sites; the difference between the frontal and frontocentral midline sites was not significant (p>0.10), but all other pairwise comparisons of caudality were significant (p<0.001). All of the pairwise comparisons of the midline sites with all other laterality sites were also significant (p<0.001). Moreover, the depressed group showed higher amplitudes of error-related negativity than the comparison subjects (group effect: F=4.6, df=1, 33, p<0.05).
Incentive conditions
Figure 3 depicts waveforms for each condition at a representative site, FCz. In the incentive conditions, the amplitude of error-related negativity was also greatest at the frontal and frontocentral sites (all pairwise comparisons of caudality and laterality, p<0.01). In addition, the depressed group exhibited greater error-related negativity than the comparison group (group effect: F=6.3, df=1, 33, p<0.05). Moreover, the comparison and depressed subjects showed different patterns of error-related negativity after errors in the incentive conditions (incentive-by-group interaction: F=4.6, df=1, 33, p<0.05). Parsing of this interaction revealed that the depressed individuals showed greater error-related negativity than the comparison subjects, particularly in the punishment condition (F=7.6, df=1, 33, p<0.01). In the reward condition, the depressed participants showed a pattern suggesting greater error-related negativity than in the comparison subjects (group effect: F=2.1, df=1, 33, p=0.16). Within the comparison subjects, error-related negativity in the reward condition was nearly significantly greater than in the punishment condition (incentive effect: F=3.8, df=1, 16, p<0.07).
Correlations with symptoms
In the group with major depressive disorder (N=18), Pearson correlations between symptoms and the magnitude of error-related negativity in the neutral condition revealed that the magnitude increased with greater severity across all measures of depression (BDI: r=0.39, p<0.10; Beck Hopelessness Scale: r=0.56, p<0.05; Mood and Anxiety Symptom Questionnaire, depression: r=0.51, p<0.05). No relationship between error-related negativity and the Mood and Anxiety Symptom Questionnaire ratings for anxiety or anhedonia emerged (anxious arousal: r=–0.10; general anxiety: r=0.04; anhedonia: r=–0.08; all p>0.50).
In the nonpsychiatric comparison group (N=17), no statistically significant correlations were observed between error-related negativity and self-report scores (r<0.30 for all self-report measures).
Error Positivity
Neutral condition
Figure 2 shows grand average waveforms at midline and lateral sites for each group. For all participants, the amplitude of error positivity was maximal at the central and centroparietal sites. There was no significant difference between the central and centroparietal sites (p>0.10), but the differences in all other pairwise comparisons of caudality were significant (p<0.01), and the amplitude was greater at midline sites than at lateral sites (all laterality pairwise comparisons, p<0.05). Moreover, the comparison and depressed groups showed equivalent amplitudes of error positivity (group effect: F=0.03, df=1, 33, n.s.).
Incentive conditions
In the incentive conditions, the error positivity amplitudes were also greatest at sites Cz and CPz. There was no significant difference between the central and centroparietal sites (p>0.10), but the differences in all other pairwise comparisons of caudality were significant (p<0.01), and all laterality pairwise comparisons with the midline site were significant (p<0.001). The comparison and depressed groups did not differ in error positivity amplitudes (group effect: F=0.0, df=1, 33, n.s.). The incentive-by-group analysis revealed a nearly significant difference in the pattern of error positivity amplitude after errors in the incentive conditions between the comparison subjects and depressed individuals (F=3.6, df=1, 33, p<0.07). Targeted analyses of within-group effects suggest that the depressed group had greater error positivity in the reward condition than in the punishment condition (p=0.09).
Correlations with symptoms
No correlations between error positivity and scores on symptom subscales were observed in the depressed or comparison participants (for all, r<0.30).
Discussion
To our knowledge, the present study is the first to show greater response-locked error-related negativity in individuals in a current major depressive episode compared with nonpsychiatric participants. As we will discuss, these data inform current biological, cognitive, and clinical conceptualizations of depression.
Studies implicating the anterior cingulate cortex as a probable neural generator of error-related negativity (e.g., 25–27, 36) also provide experimental evidence that paralimbic regions, including the anterior cingulate cortex, recruit activity in executive brain areas that modulates affective and behavioral responses when actual and intended goal states differ (e.g., 37, 38). In the context of the current study, enhanced error-related negativity in major depressive disorder may indicate the initial recruitment of excessive error-detection processes that then exaggerate the neural and cognitive resources allocated to generating subsequent adaptive responses. That is, an enhanced error-related negativity such as that observed in depression may wrongly signal that errors are large or highly significant
(38,
39) and recruit excessive behavioral, cognitive, and affective processes that manifest in the signs and symptoms associated with the disorder
(18,
21) . Of clinical relevance, the neural generators of the error-related negativity identified in source localization analyses and the brain regions identified in fMRI studies of error processing overlap with brain areas implicated in treatment response in major depressive disorder (for reviews, see references
14 and
40 ). The current data invite the possibility that enhanced error-related negativity in depression similarly reflects a generally heightened sensitivity to discrepancies between affective goal states and actual states that may serve as a promising prognostic indicator of the course of illness and response to treatment.
The enhanced error-related negativity observed in the depressed group also contributes to a growing literature investigating affective influences on error-related negativity (e.g.,
41,
42) . Specifically, whereas enhanced error-related negativity has been observed in individuals with high levels of negative affect (e.g.,
41,
42), equivalent
(28) or diminished
(43) error-related negativity has been observed in individuals with remitted depression. To date, two studies have examined a related medial frontal negativity of the event-related potential that is sensitive to negative feedback. This “feedback-related negativity” appears to be enhanced in currently depressed individuals
(3) and diminished in those whose depression has remitted
(44) . In the present study, enhanced amplitude of response-locked error-related negativity was specifically associated with greater severity of depression, but not anxiety symptoms, across a variety of scales. The current data, in conjunction with prior reports of diminished error-related negativity in individuals with remitted depression, thus suggest that error-related negativity may be a viable index of both the presence of a major depressive episode and the severity of depression-specific symptoms.
The robustness of the enhanced error-related negativity in major depression was evident across all conditions. In addition, the depression and comparison groups showed differences in the sensitivity of error-related negativity to motivating incentives. In particular, the depressed group, relative to the comparison group, demonstrated enhanced error-related negativity, particularly in the punishment condition, whereas the comparison subjects appeared to show greater error-related negativity in the reward condition than in the punishment condition. These data indicate that neural resources involved in early error detection are susceptible to behavioral reinforcers in a manner consistent with prior reports in depression. In particular, this pattern of error-related negativity modulation is consistent with the substantial evidence indicating excessive sensitivity to loss and failure and diminished response to hedonic cues among individuals with major depressive disorder (e.g.,
1 –
5,
10 –14) .
While group differences were observed in the magnitude of error-related negativity, equivalent error positivity amplitudes were noted in the depressed and comparison groups. The distinct pattern in the depressed group of a normal overt behavioral response followed by exaggerated error-related negativity and intact error positivity further implicates early error detection, rather than subsequent recognition processes, in the hypersensitivity to negative cues seen in the disorder. The depressed group also showed nonsignificantly greater error positivity in the reward condition than in the punishment condition, whereas the nonpsychiatric participants exhibited no differences in error positivity amplitude between the two conditions. The patterns of error-related negativity and error positivity in the reward and punishment conditions, coupled with the absence of group or incentive differences in overall reaction time and accuracy, suggest that the neural and psychological responses reflected in error-related negativity and error positivity represent processes serving complementary functions that are differentially engaged to produce intact behavioral responses. In support of this conjecture, post hoc analyses revealed a nearly significant negative correlation between the amplitudes of error-related negativity and error positivity such that greater error-related negativity was associated with a lower amplitude of error positivity in individuals with depression (r=0.44, N=18, p<0.10).
Limitations
The caveats regarding the current work provide impetus for future research. First, as the primary aim of this work was to test whether enhanced error-monitoring characterizes major depressive disorder, the task implemented in the present study was chosen explicitly to restrict task demands as much as possible to processes related to error monitoring. In comparison, prior studies have used neuropsychological tasks in which the participant is likely to be unaware of his or her accuracy until feedback is presented
(6 –
8) . Thus, while the design and simple nature of the flanker task allowed the examination of specific error-processing mechanisms, the differences between the designs of the present study and other investigations render direct comparisons difficult. In addition, as both error-related negativity and error positivity have previously been observed to attenuate over time as participants’ inherent motivation toward the task diminishes
(22,
42), the block design implemented in the current work may have reduced potential modulatory effects of task contingencies on physiological measures of error processing.
Summary
The current work examined component processes of error monitoring in individuals with current major depressive disorder and the effects of task incentives on these processes. Most striking, individuals with major depression exhibited greater magnitude of error-related negativity than did nonpsychiatric comparison subjects. In addition, task incentives appeared to differentially modulate the error-related negativity and error positivity for the two groups. Together, these data implicate exaggerated early error-detection processes in the etiology and maintenance of major depressive disorder. These processes may then recruit excessive neural and cognitive resources that manifest as symptoms of depression.