The results of this study demonstrate that cocaine dependence is associated with a reduction in D 2/3 receptor availability and a decrease in amphetamine-induced dopamine release in the ventral striatum and putamen. In the cocaine-dependent volunteers, the decrease in amphetamine-induced dopamine release correlated with the choice for cocaine in the self-administration sessions, such that participants with the greater impairment in presynaptic dopamine in the anterior caudate and ventral striatum were the more likely to choose cocaine over an alternative reinforcer. Insofar as the self-administration sessions provide a valid predictor of relapse, these findings suggest that impairment in presynaptic dopamine function plays a critical role in maintaining the habitual, maladaptive patterns of behavior that are indicative of addiction.
Dopamine Presynaptic Transmission
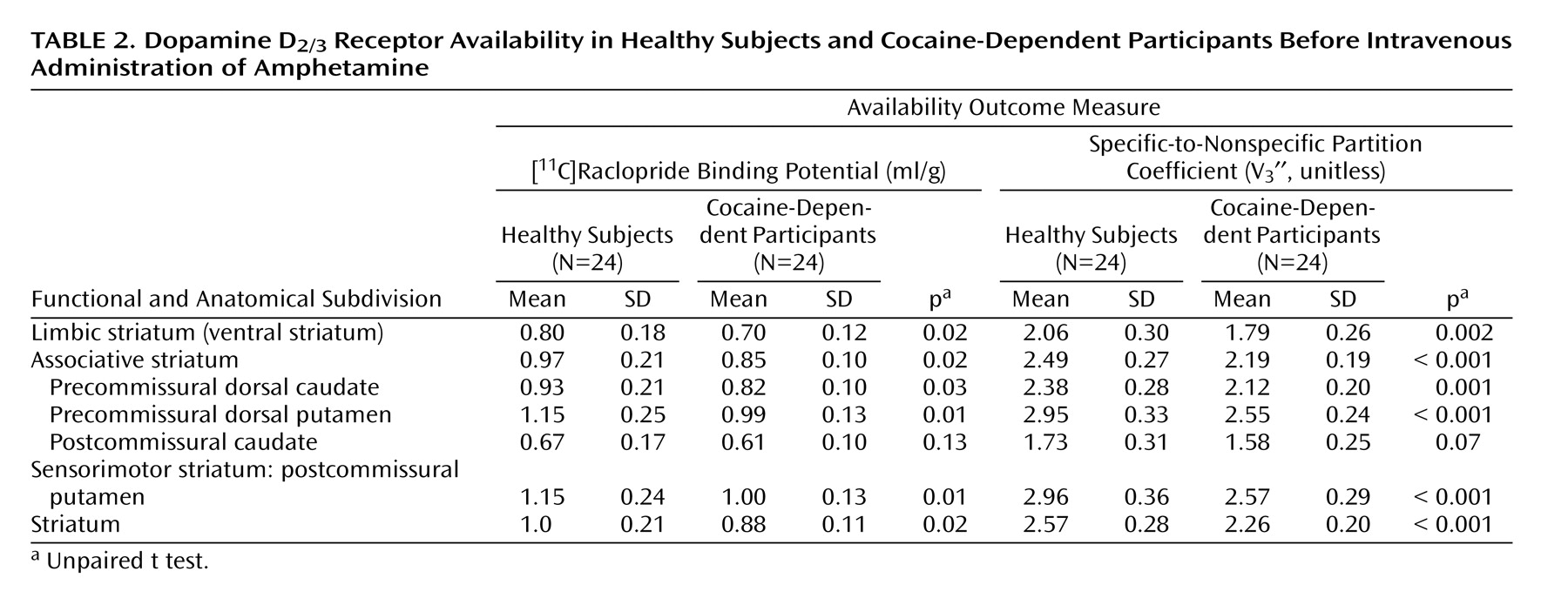
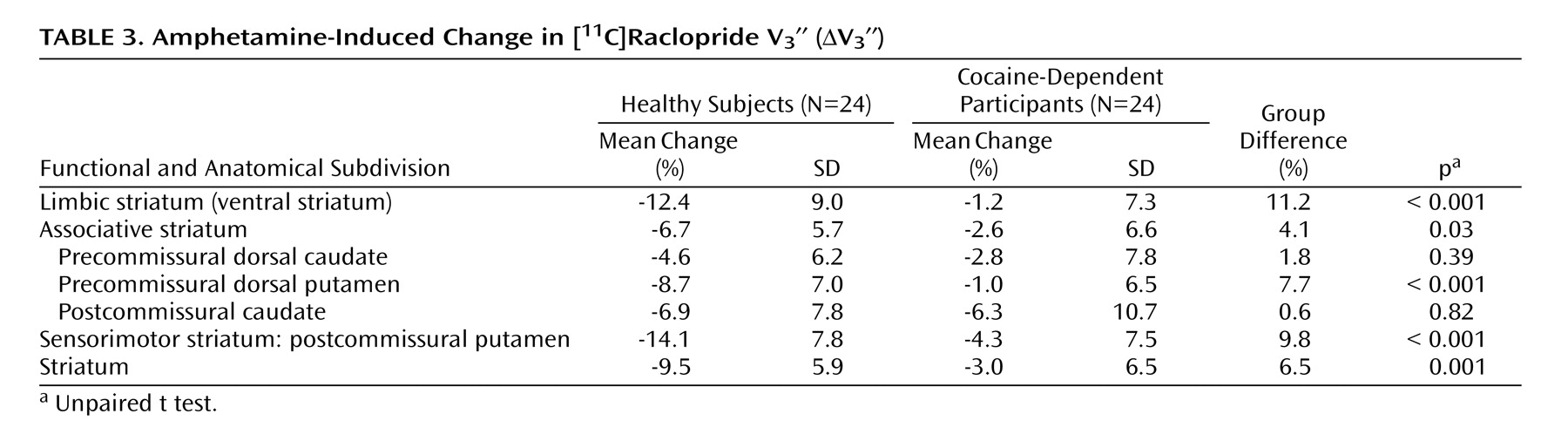
In contrast to the modest magnitude of the decrease in D 2/3 receptor availability, the deficit in presynaptic dopamine function in the cocaine-dependent participants was severe. Relative to healthy subjects, cocaine-dependent participants showed a 90%, 61%, and 70% reduction in ΔV 3 ′′ in limbic, associative, and sensorimotor regions, respectively. This reduction was significantly greater in the limbic striatum than in the associative striatum, but not significantly different from the sensorimotor striatum. In other words, we did not support our hypothesis that cocaine dependence would be associated with a greater reduction in the limbic striatum. Instead, we demonstrated a similar decrease in the limbic and sensorimotor striatum, suggesting that cocaine dependence is associated with a prominent motor component in addition to changes in limbic dopamine transmission.
With respect to the individual regions of interest, there was a significant group difference in the ventral striatum and putamen but not the caudate. This might be related to the fact that the effect of amphetamine on [
11 C]raclopride V
3 ′′ is low in the caudate of healthy subjects
(11,
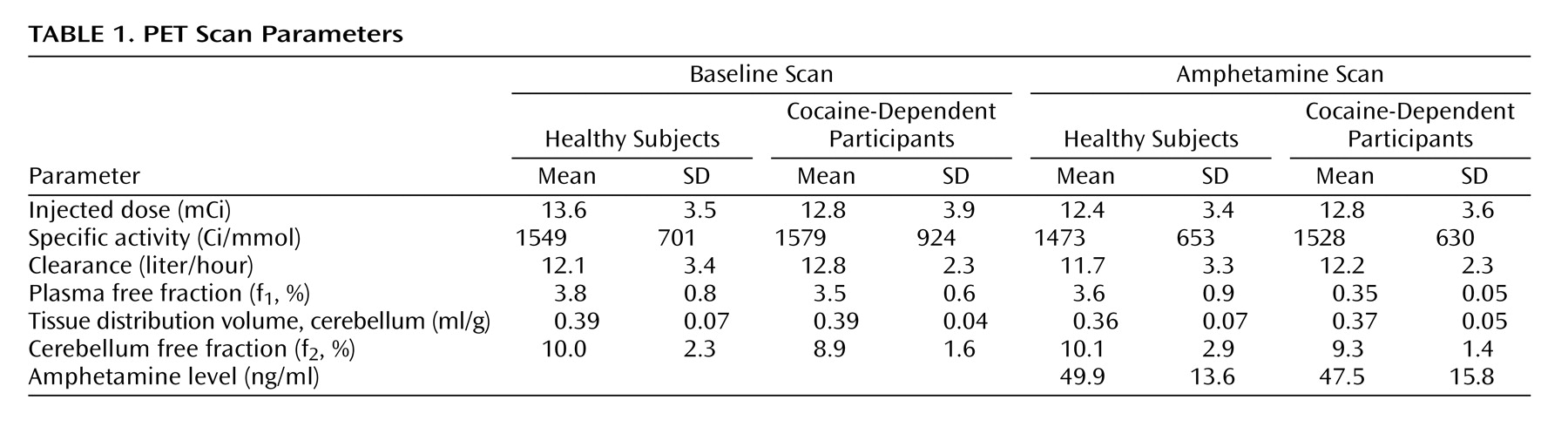
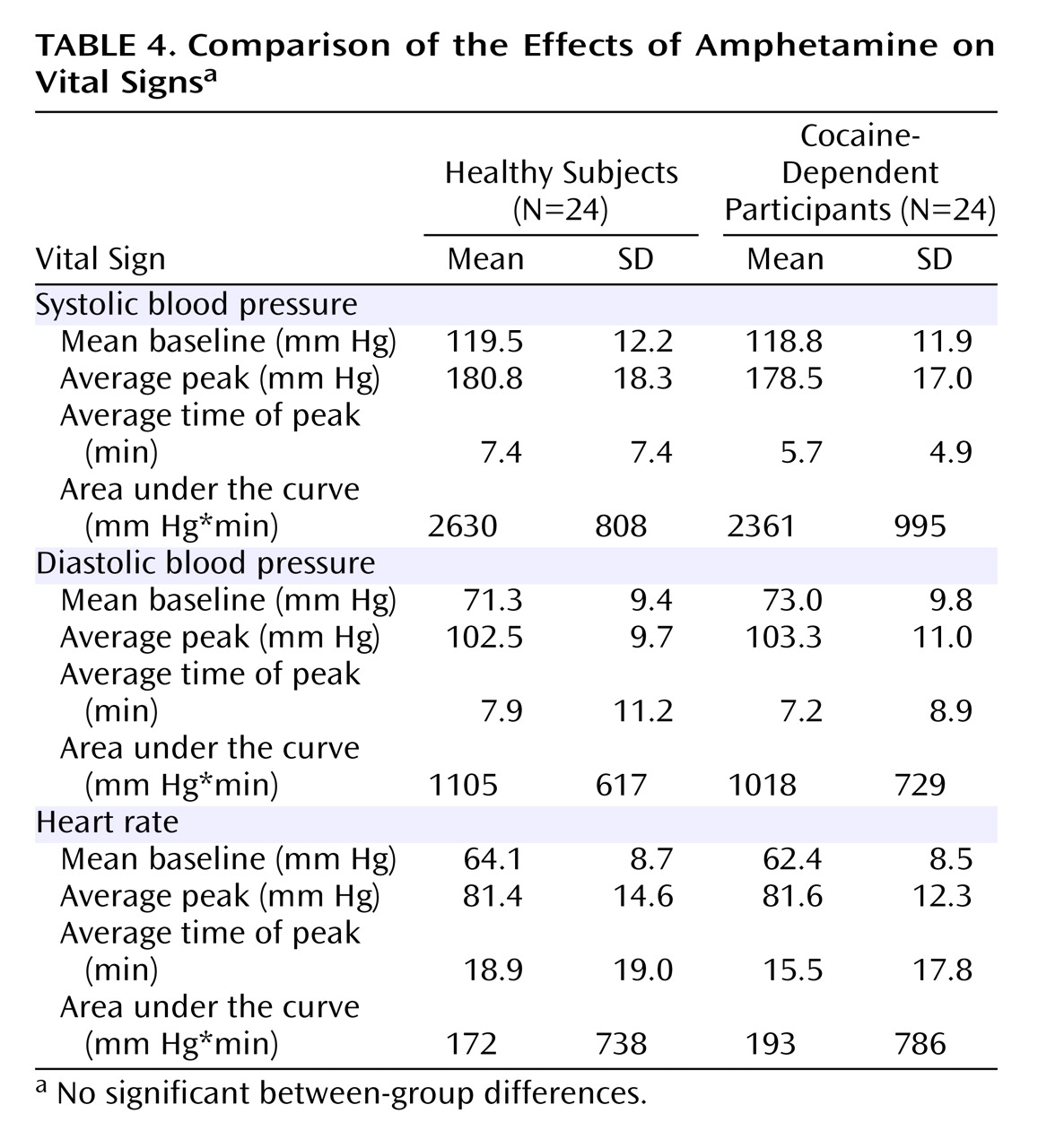
13,
19) . The blunted dopamine response to amphetamine in cocaine-dependent participants could not be attributed to pharmacokinetic differences in amphetamine, since there was no difference in amphetamine plasma levels or vital signs between the two groups. Nevertheless, the decrease in amphetamine-induced dopamine release in cocaine abusers in this study is in agreement with Volkow et al.
(2), who showed a similar reduction in [
11 C]raclopride displacement in the striatum as a whole following intravenous methylphenidate (0.5 mg/kg).
The results of these imaging studies are in contrast to preclinical studies demonstrating sensitization of the dopamine response in the striatum to stimulants in laboratory animals exposed to cocaine
(20 –
22) . In order for sensitization to occur, the animal must undergo exposure to cocaine followed by a period of abstinence. Given these findings, it would be expected that chronic cocaine abusers administered a psychostimulant would show an excess of dopamine release rather than a blunted effect. The study of Volkow et al.
(2) and our study were performed on participants who had been abusing cocaine for prolonged periods of time and the scans were performed following a period of abstinence (3–6 weeks in the study of Volkow et al. and 14 days in our study), such that dopamine sensitization should have been elicited. Therefore, these studies suggest that dopamine sensitization may not be present in humans who have been exposed to cocaine for several years.
The decrease in dopamine transmission is consistent with the observations that, in rodents, chronic cocaine exposure is associated with a reduction in axonal transport of dopamine to the striatum as well as a decrease in cocaine-induced dopamine release
(23 –
26) . Postmortem studies in humans have shown that cocaine dependence is associated with a decrease in the levels of vesicular monoamine transporter type 2, which may represent a decrease in or injury to the midbrain dopamine neurons
(27,
28) . Furthermore, Wu et al.
(29) demonstrated a reduction in [
18 F]6-FDOPA uptake, which provides a measure of dopamine synthesis in the axon terminals of the striatum in abstinent cocaine abusers. Together, these studies support the hypothesis that cocaine dependence is associated with a loss of presynaptic dopamine transmission.
In this context, it is important to mention that Volkow et al.
(2) reported a greater response to methylphenidate in the thalamus in the cocaine-dependent volunteers (29% versus no effect in healthy subjects), suggesting sensitization may occur in this brain region. Based on the finding of Volkow et al., we performed an exploratory analysis of this region but the difference between cocaine-dependent and healthy subjects did not reach significance (ΔV
3 ′′=5.2% [SD=18.3%] and 3.2% [SD=16.8%], respectively; p=0.70). No correlation was seen between dopamine release in the thalamus and the choice for cocaine, positive effects of cocaine, or subjective effects of amphetamine.
Dopamine Transmission and the Choice to Self-Administer Cocaine
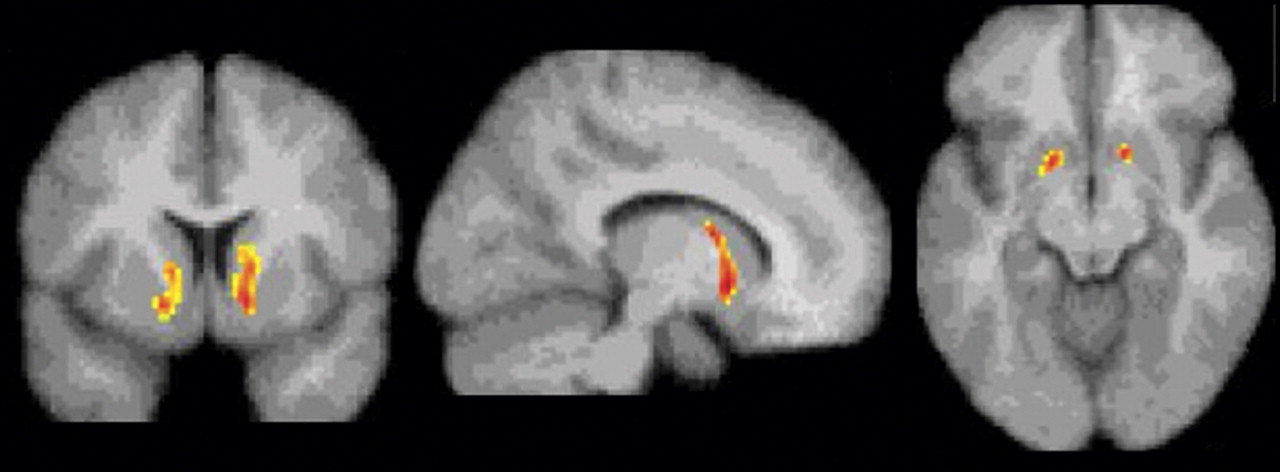
The deficit in dopamine function in the ventral striatum and anterior caudate was predictive of the choice for cocaine over a monetary reward in the laboratory setting. Insofar as these sessions serve as a model of relapse, this finding suggests that cocaine-dependent subjects who are the most vulnerable to relapse are those with the lowest presynaptic dopamine function. This finding was limited to the ventral striatum in the voxel-based analysis. However, a region of interest analysis of ΔV
3 ′′ and the choice for cocaine demonstrated a significant negative association in the caudate rostral to the anterior commissure in addition to the ventral striatum. It should be noted that amphetamine, rather than cocaine, was chosen for challenge because amphetamine can be administered to healthy subjects and since the correlation between dopamine and radiotracer displacement has been established in microdialysis studies on nonhuman primates
(30,
31) . Nevertheless, future studies using a cocaine challenge would be of interest, since cocaine and amphetamine have different mechanisms of action.
The importance of striatal dopamine transmission in brain reward mechanisms has been established through numerous studies
(32) . In nonhuman primates, dopamine has been described as encoding a “reward prediction signal” which plays a critical role in discriminating between competing rewards
(33) . Dopamine has also been described as mediating the “incentive salience” of a reward
(34) or mediating the behavioral economics of reward
(35) . These studies suggest that dopamine serves to modify goal directed behavior, as originally hypothesized by Bindra
(36) .
Thus, dopamine may provide the signal that emphasizes a particular reward, even at the expense of a competing reward. This hypothesis is in agreement with the present study. In the self-administration sessions, participants were given the choice between low-dose cocaine and a voucher worth $5, both of which serve as reinforcers. However, the doses of cocaine had a street value of less than $5, so that the choice options were weighted toward the money. The failure of the dopamine-depleted cocaine-dependent participants to alter their behavior suggests that these individuals have the greatest deficit in their ability to shift between reward-directed behaviors. We propose that, in this setting, dopamine transmission is critical for making the shift from a habitual behavior that carries a lesser reward (the choice for cocaine) to a more adaptive behavior that is associated with a greater (albeit delayed) reward (the choice for money). The loss of dopamine may represent a loss in the ability to shift from the habitual behavior to another behavior, even one that is associated with a reward. Ultimately, this loss of dopamine may underlie the inability of the individuals with refractory cocaine addiction to respond to therapeutic attempts to substitute more adaptive rewards for habitual cocaine use. Future studies are needed to address this question.