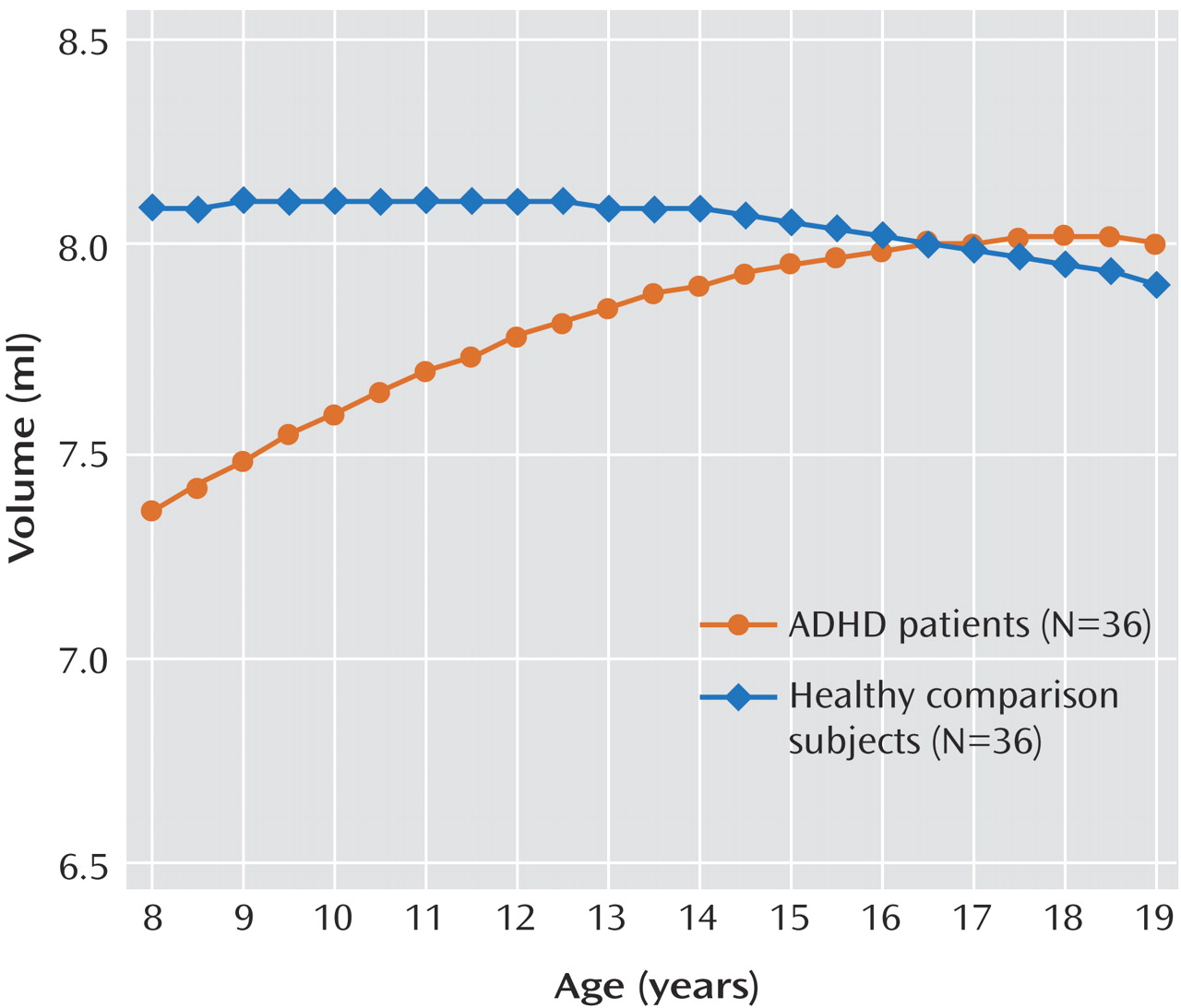
There has been increasing interest in the role of the cerebellum in the pathogenesis of ADHD. Structural anomalies of the cerebellum are among the most consistently reported features of ADHD, notably reductions in overall volume
(2), particularly in the cerebellar vermis
(3 –
7) . Diffusion tensor imaging of cerebellar white matter has revealed that attentional impairment is associated with decreased fractional anisotropy in this region
(8) . Although the cerebellum has traditionally been considered a site of motor control, lesion studies and functional imaging studies in healthy subjects have demonstrated a wide range of cognitive and affective functions in cerebellar structures, including temporal information processing, shifting attention, verbal working memory, implicit learning, executive function, and emotional regulation
(9 –
12) . Dysfunction in each of these cognitive domains has in turn been implicated in the etiology of ADHD
(13,
14) .
We reexamined longitudinal data from a sample of 36 children with ADHD and 36 children without ADHD, all of whom underwent neuroanatomic magnetic resonance imaging (MRI) scans on at least three separate occasions. The current study included 157 scans from the children for whom we previously reported changes in total cerebellar volume
(2) and an additional 72 scans acquired since that time, for a total of 229 scans. We measured the three hemispheric lobes and the corpus medullare on each side as well as three lobes of the midline vermis. We hypothesized that we would replicate the finding of smaller overall cerebellar volumes reported in previous structural imaging studies, and we expected to refine previous findings of a smaller midsagittal area in the inferior vermis
(3 –
7) through a novel method of volumetric analysis. Given evidence regarding regional cerebellar activation during attention tasks in functional studies
(16), we also anticipated prominent findings in the posterior hemispheres. By adopting a longitudinal design, we further aimed to characterize the developmental trajectories of individual hemispheric and vermal lobar volumes in relation to the diagnosis of ADHD and in relation to long-term clinical outcome. In light of recent findings relating clinical outcome to abnormalities in the prefrontal cortex
(17), we hypothesized that volume normalizes in some regions of the cerebellum as disease symptoms diminish, thus distinguishing groups with better and worse outcomes.
Method
Participants
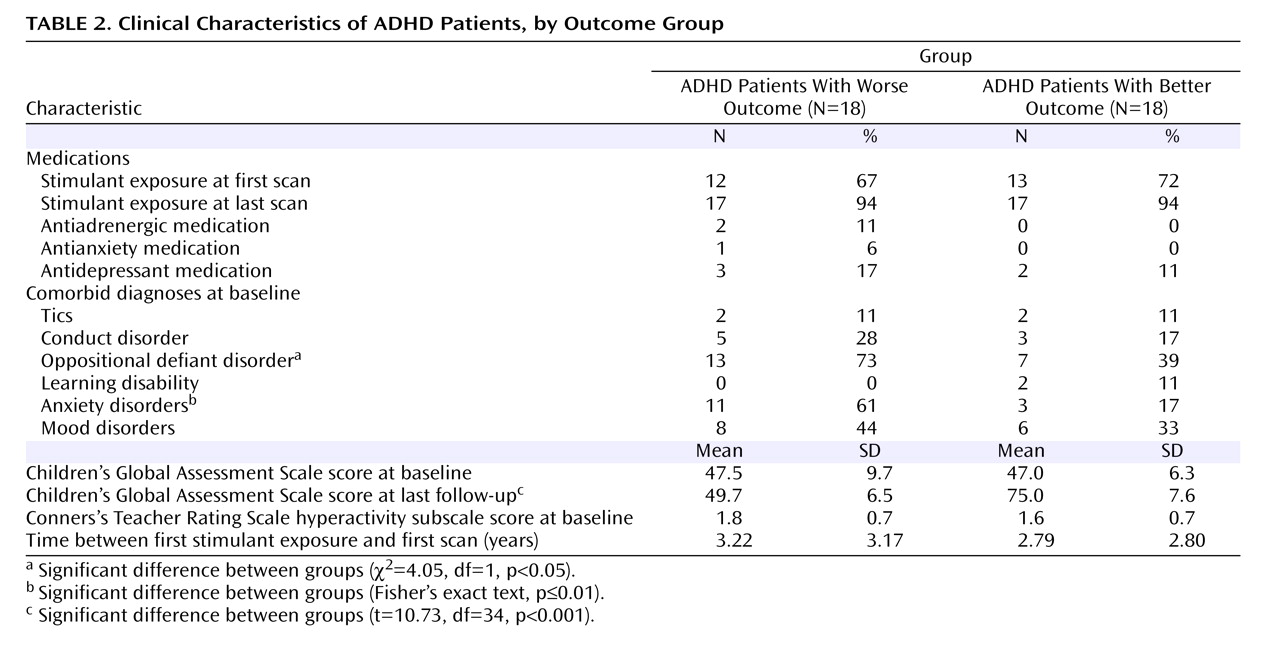
Thirty-six children and adolescents who met DSM-IV criteria for ADHD and for whom at least three usable MRI scans were available were selected from a larger sample of participants recruited for imaging studies at the National Institute of Mental Health (NIMH). Recruitment details have been described elsewhere
(2) . Diagnoses were made on the basis of the Diagnostic Interview for Children and Adolescents
(18) and a rating on the hyperactivity subscale of the Conners’s Teacher Rating Scale greater than two standard deviations above age- and sex-specific mean ratings
(19) . Exclusion criteria were a full-scale IQ score below 80 and evidence of medical or neurological disorders. Comorbid psychiatric diagnoses among participants were relatively mild and were not the focus of treatment in any case. Thirty-five participants had combined-type ADHD (97%), and one (3%) had the hyperactive subtype. Fourteen participants (39%) underwent four scans, and one (3%) underwent five scans.
Thirty-six unrelated comparison subjects who had no personal or family history of psychiatric or neurological disorders were recruited from the community as part of the ongoing recruitment of the Child Psychiatry Branch at NIMH. Matched selection was used to minimize group differences in IQ, socioeconomic status, gender, and handedness. All subjects had undergone at least three scans; 18 (50%) had four scans, four (11%) had five scans, and one (3%) had seven scans.
The NIMH institutional review board approved the research protocol, and written informed consent and assent to participate in the study were obtained from the parents and children, respectively.
Clinical Outcome Measures
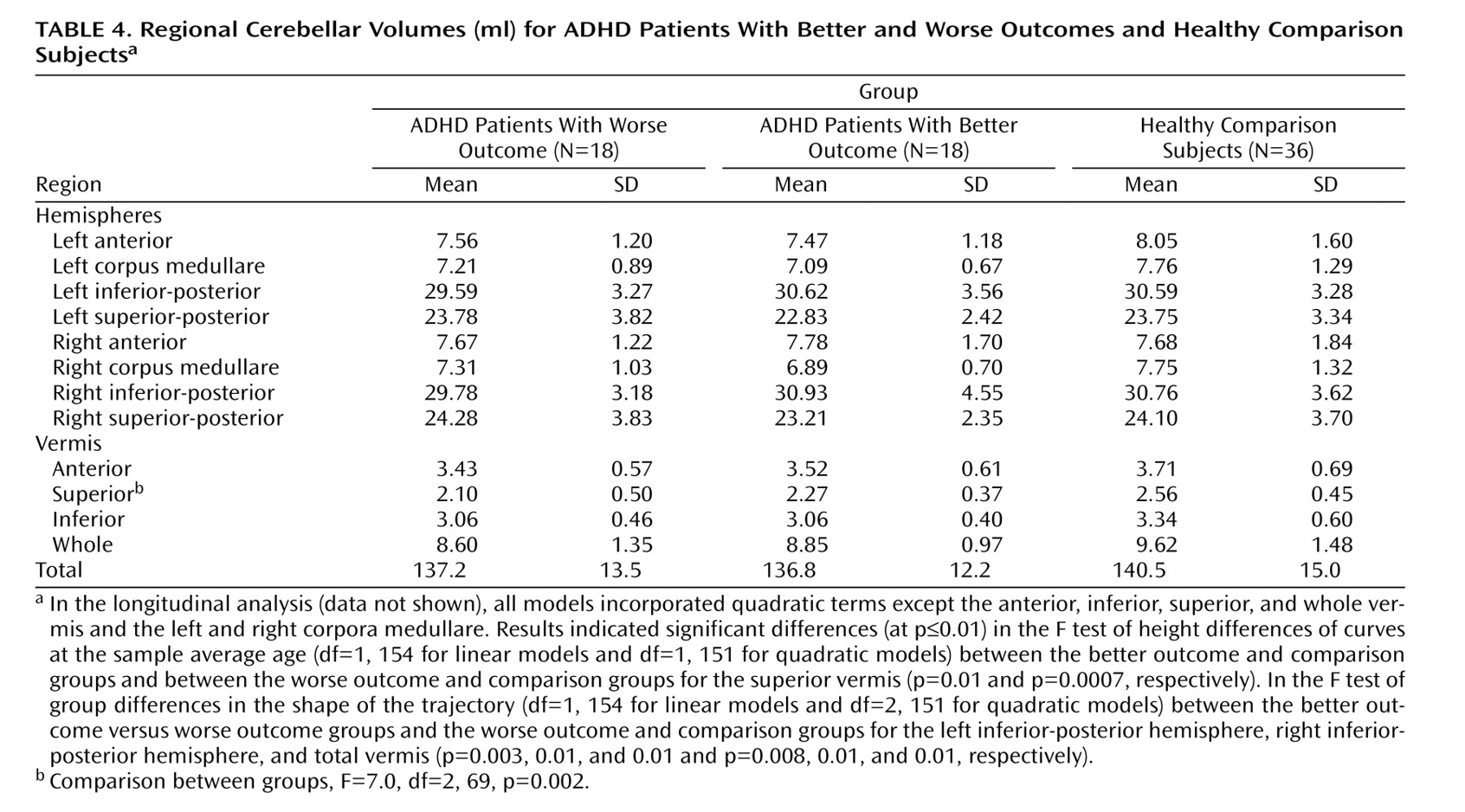
The last available score on the Children’s Global Assessment Scale (CGAS)
(20) was used to characterize clinical outcome. Patients with ADHD were divided into groups with better (N=18) and worse (N=18) outcomes on the basis of the mean final follow-up CGAS score, which was 62.4 (SD=14.6); a better outcome was defined as a CGAS score ≥62 and a worse outcome as a CGAS score <62. CGAS scores, symptom scores, and diagnostic data were available for all ADHD patients at baseline and at the last follow-up. Clinical assessments were performed independently of neuroimaging analyses. The mean ages of the groups at each wave of the assessment and MRI scanning did not differ significantly. At the last follow-up, patients were further categorized according to DSM-IV criteria as having inattentive subtype only (N=11), hyperactive/impulsive subtype only (N=2), or combined type (N=12) or as having attained full remission (N=10).
Neuroanatomic Analyses
T1-weighted images with contiguous 1.5-mm sections in the axial plane and 2.0-mm sections in the coronal plane were obtained using three-dimensional spoiled gradient recalled echo in the steady state on a Signa 1.5-T scanner (General Electric Medical Systems, Milwaukee, Wisc.) (echo time=5 msec; repetition time=24 msec; flip angle=45°; acquisition matrix=256×192; number of signals acquired=1; field of view=24 cm). The original MR images were registered into standardized stereotaxic space using a linear transformation and corrected for nonuniformity artifacts
(21) . An advanced neural net classifier was used to segment the registered and corrected volumes into white matter, gray matter, CSF, and background
(22) .
Gray and white matter volumes of the total cerebrum were quantified using an automated technique developed at the Montreal Neurological Institute that combines voxel-intensity based tissue classification into gray matter, white matter, and CSF with a probabilistic atlas
(23,
24) . The cerebellum could not be classified owing to the complexity of its gray-white border.
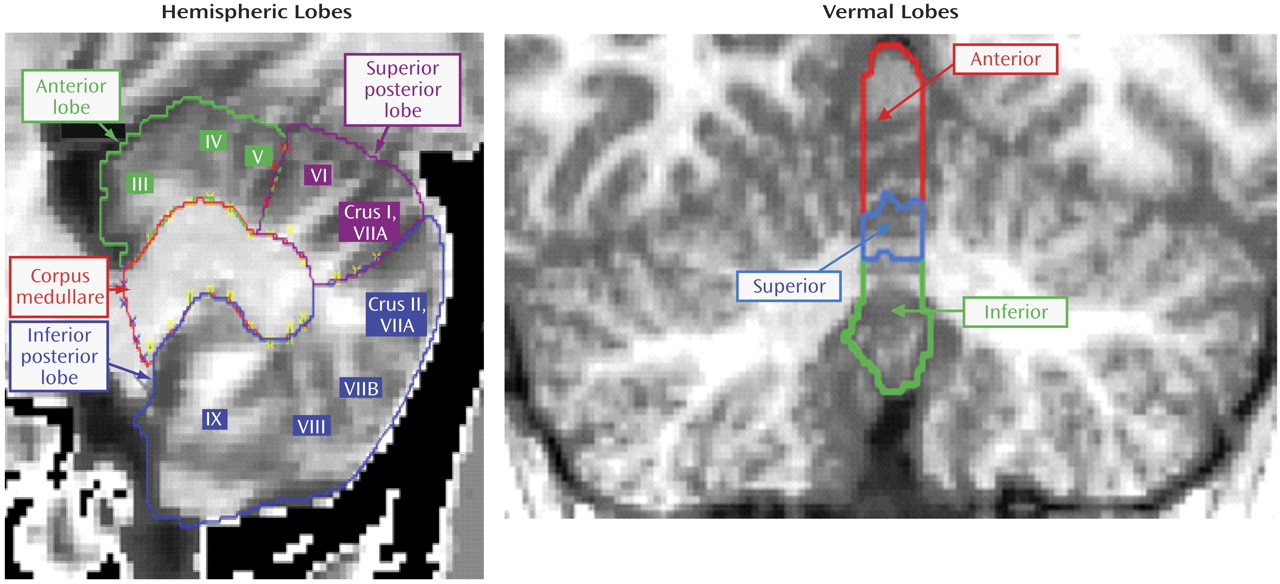
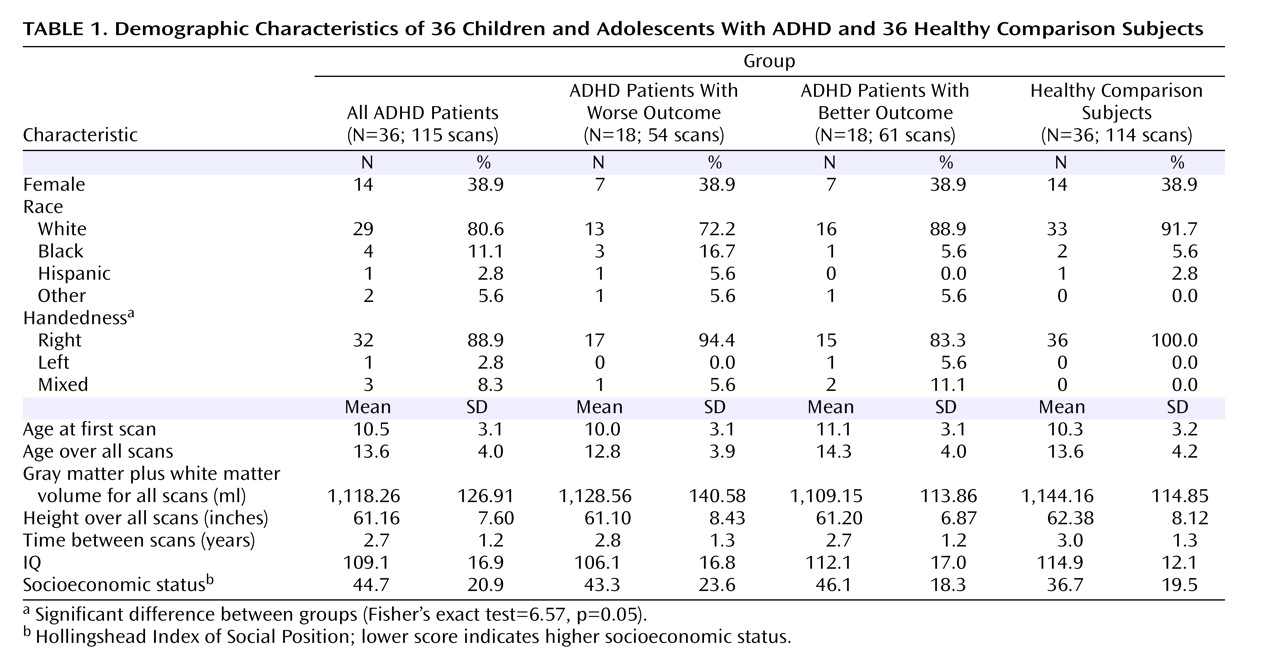
The cerebellar parcellation method developed by Pierson and colleagues at the University of Iowa
(15) was used in conjunction with the BRAINS2 software package
(25) to generate outlines of each lobe of the cerebellar cortex and corpus medullare. Briefly, 31 landmarks were manually defined and used as the basis for the application of a neural net to generate surface masks of each of the cerebellar subregions. These were then reviewed and edited manually. Volumes of each subregion were measured and summed to provide the total cerebellar volume.
Masks of vermal subregions were manually traced. A midline guide trace in the sagittal plane marked the superior, inferior, anterior, and posterior limits of the vermis, and the axial plane was used to generate guide traces approximating the lateral borders. The vermis was defined in each coronal slice by manual tracing with reference to these guide traces. Regional subdivision was accomplished through placement of limiting boundaries in the sagittal plane, where the primary and prepyramidal fissures were readily apparent throughout the vermis.
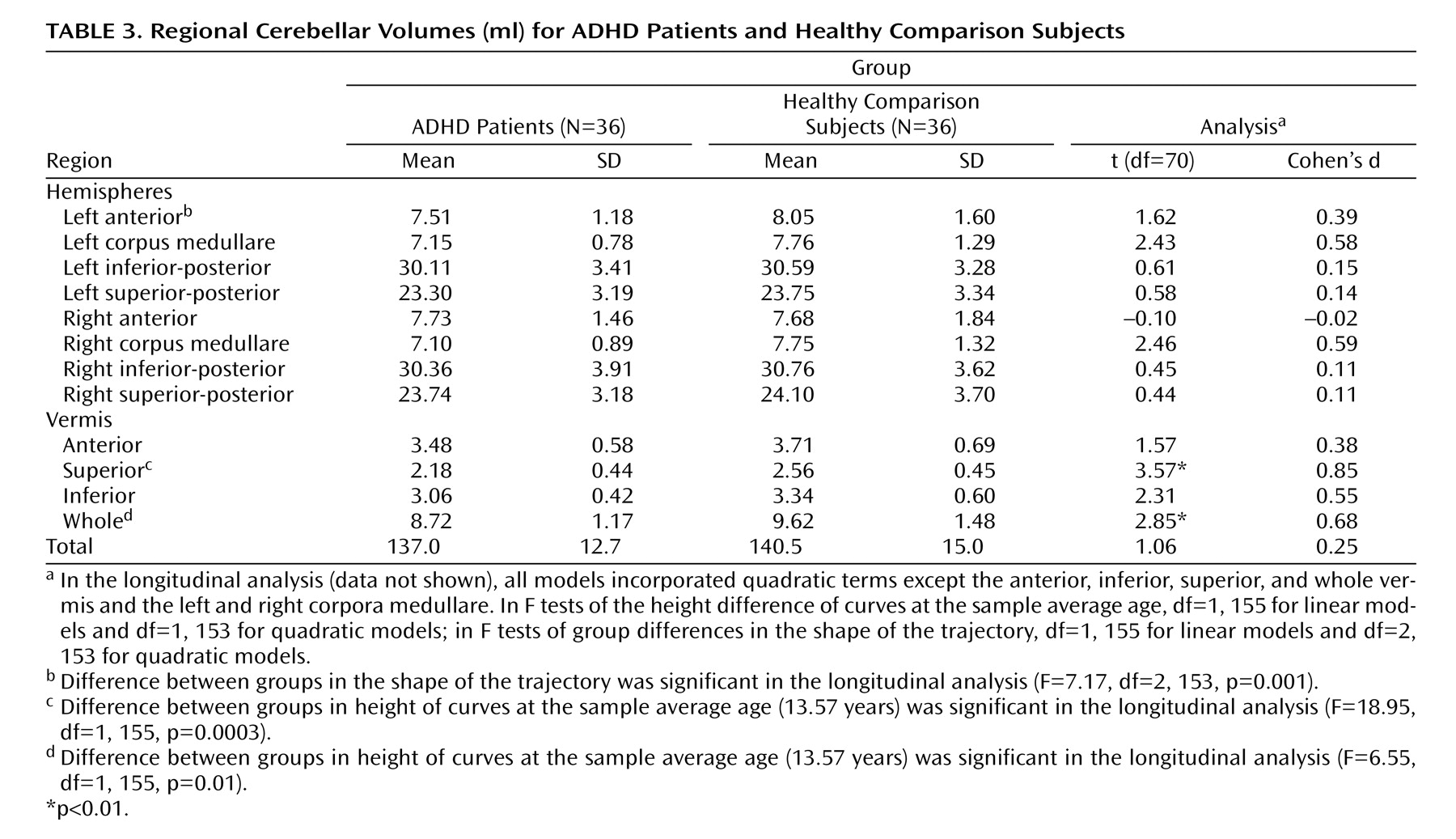
The left and right cerebellar cortices were each divided into anterior, superior-posterior, and inferior-posterior lobes using the nomenclature of Larsell and Jansen
(26) . The anterior lobe was composed of lobules I–V and delineated by the primary fissure. The superior-posterior lobe was defined to include lobules VI and VIIA-folium in the vermis region and lobule VI and crus I of VIIA in the hemispheres. It was separated from the inferior-posterior lobe by the horizontal fissure. The inferior-posterior lobe consisted of the lobules VIIA-tuber through X. The three hemispheric lobes consist predominantly of gray matter, although white matter branching off into the folia was included in the lobes. The left and right corpora medullare were also defined, including all of the central region, which appears as white matter. The MRI acquisition method used for this study was not able to visualize or separate out the deep nuclei within the corpus medullare. The vermis was divided into anterior, superior, and inferior regions. The primary fissure determined the posterior extent of the anterior lobe, and the prepyramidal fissure separated the superior from the inferior lobe. These regions are illustrated in
Figure 1 .
Intraclass correlations (ICCs) for all hemispheric regions and the corpus medullare were >0.80 for both intrarater and interrater reliability. In the vermis, intrarater reliability ICCs were all >0.80, and interrater ICCs were all >0.68.
Statistical Analysis
Differences between groups were assessed by t tests and analysis of variance (ANOVA) for continuous measures and chi-square tests and Fisher’s exact test for categorical measures. Mixed-model polynomial regression was used to examine cerebellar development. This method was chosen over traditional methods, such as repeated-measures ANOVA, because it permits the inclusion of multiple measurements per person, missing data, and irregular intervals between measurements, thereby increasing statistical power. For the polynomial comparisons between ADHD and comparison groups, the i th individual’s j th cerebellar volume was modeled as follows:
Volume ij = intercept + β 1 (diagnosis=ADHD) + β 2 (age – mean age) + β 3 (age – mean age) 2 + β 4 (diagnosis=ADHD × [age – mean age]) + β 5 (diagnosis=ADHD × [age – mean age] 2 ) + d i + e ij
where the intercept and b terms are fixed effects, d i is a normally distributed random effect that models within-person dependence, and e ij represents the usual normally distributed residual error. F values determined whether polynomial terms significantly contributed to the explanatory power of a model. After the order of the model was determined (linear or quadratic), F or t tests were used to determine whether the diagnostic curves differed in shape (that is, whether the coefficient[s] for age terms differed between groups) and height (group difference in expected volume at the average age). Parameter estimates of the fixed effects were used to generate fitted values in the graphs. Similar analyses were conducted for the three group comparisons. Fitted lines spanned the middle 80% of the data.
In view of the multiple statistical tests conducted, we adopted an alpha threshold of 0.01. Cohen’s d was used to calculate effect sizes
(27) .
Discussion
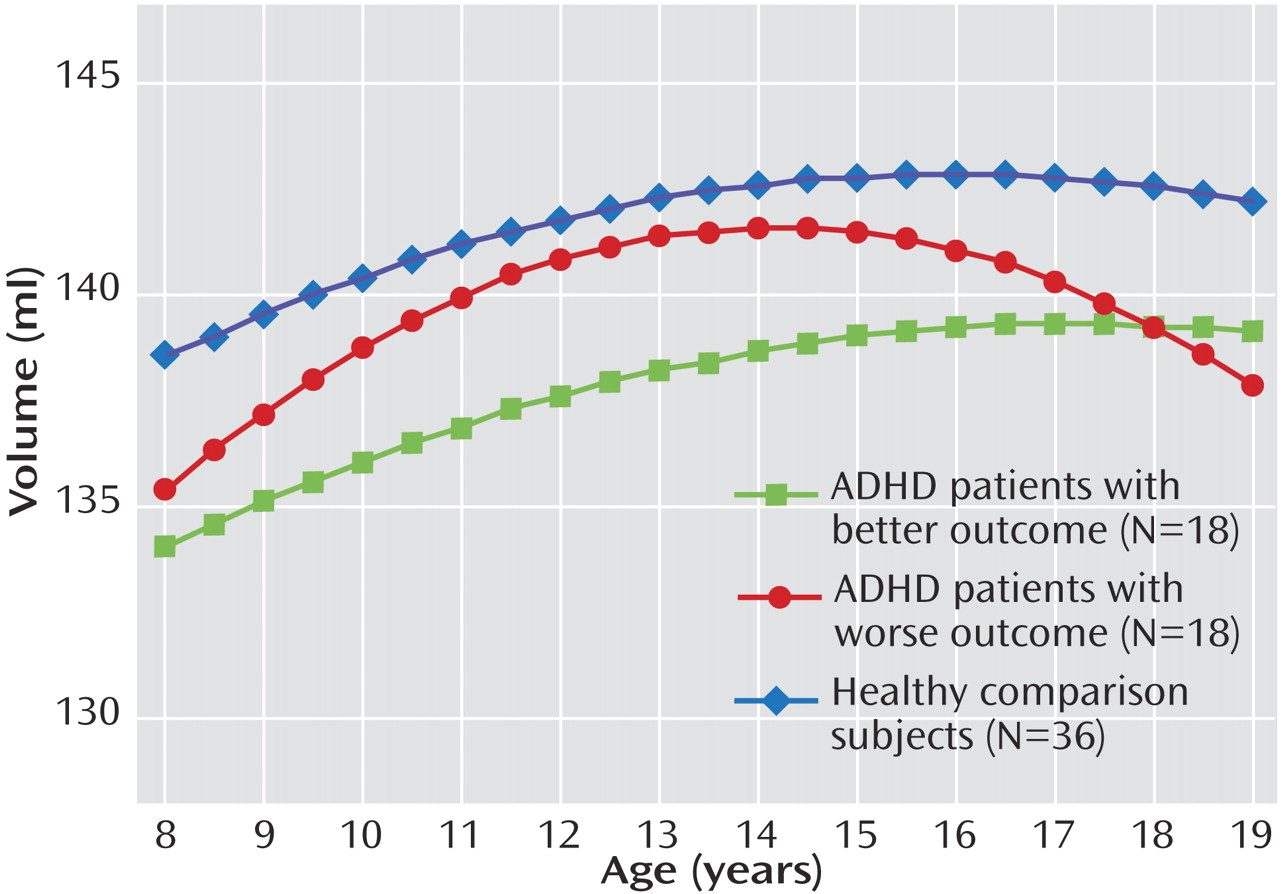
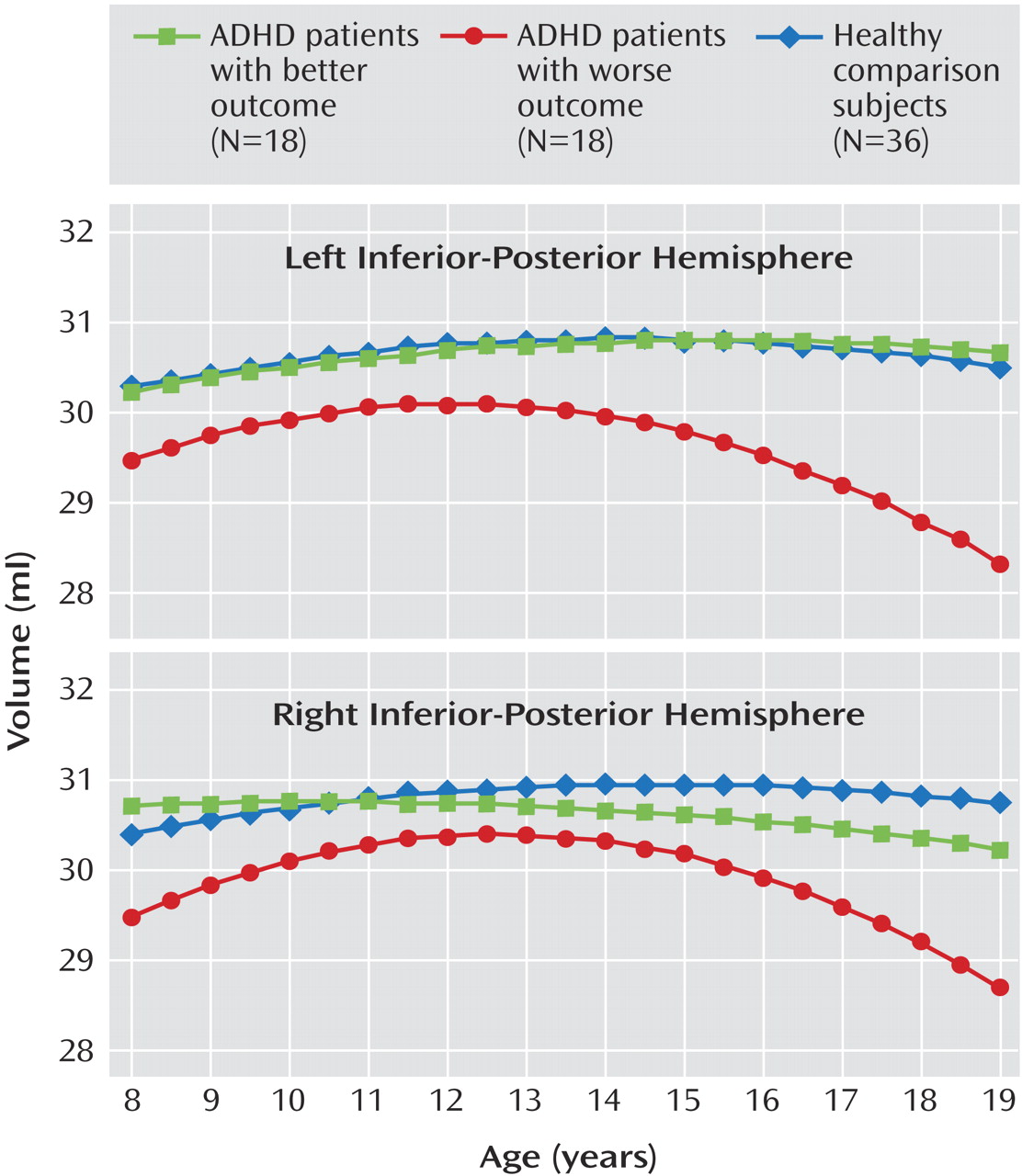
This is the first structural imaging study of ADHD to delineate the development of each lobe of the cerebellar hemispheres using longitudinal data and a volumetric regional measure of the cerebellar vermis. Using both a new semiautomated method for analyzing hemispheric lobes and a new manual method for accurately quantifying vermal subdivisions, we detected a highly specific abnormality—a smaller superior vermis—that has not previously been detected using whole cerebellar volumes or midsagittal vermal areas. Furthermore, the longitudinal design and clinical outcome stratification allowed us to demonstrate hemispheric differences between better and worse outcome groups, with the worse outcome group deviating progressively with age. These findings emphasize the mixture of fixed and progressive neuroanatomic deficits in ADHD
(2,
17) . The former may reflect a fundamental disease phenotype, and the latter may represent plastic anatomic changes that are related to symptom status.
In addition to its role in motor control, the cerebellum contributes to a wide range of cognitive and affective processing. Lesion studies demonstrate critical roles for the cerebellum in motor and perceptual tasks in which events span milliseconds, thus requiring exquisite temporal control
(28), in the orientation of spatial attention
(29,
30), in verbal working memory
(31), in language processing, and in affective regulation
(10,
32) . Functional imaging studies with healthy subjects similarly demonstrate cerebellar activation in wide range of cognitive tasks
(12,
33,
34) . Precise localization of function within the cerebellum has proved challenging given the limits of functional imaging in the posterior fossa, the heterogeneity of cerebellar damage among patients with lesions, and the possibility that cognitive functions, such as verbal working memory, require the coordinated action of multiple cerebellar regions
(31) . While this complicates interpretation of our more regional findings, some considerations are relevant.
The nonprogressive deficit localized in the superior vermis in ADHD patients in our study may represent a neuroanatomic basis of fundamental deficits in cognitive and affective processing that are resistant to plastic developmental changes in ADHD. Lesion studies have found additionally that the degree of hypoplasia in the cerebellar superior vermis is selectively associated with the severity of deficits in attention-orientation
(30) . Similarly, in studies of superficial siderosis
(10,
35) —an acquired neurological disorder of hemosiderin deposition particularly affecting the superior cerebellar vermis—deficits were identified in speech production, visual recall, executive impairments, and social comprehension; Schmahmann
(10) emphasized the latter in his clinical description of socioemotional dysregulation among patients with vermal damage. Functional imaging studies also implicate the vermis in complex cognitive activities, including time estimation
(36), perceptual processing of stimuli at short intervals
(37,
38), and the alerting and executive control aspects of attention
(39) . Deficits in time information are consistently found in ADHD, including problems with motor timing, time discrimination, and time reproduction
(40 –
44) . These deficits may lead to failures in appreciating the temporal structure of the environment, which could underpin many symptoms of ADHD
(14) . For example, impulsive behaviors in a child with ADHD may represent a failure to produce the precise timing required to participate in social exchanges. Similarly, transient lapses in attention and response variability (noted most clearly in neuropsychological testing) may also reflect an impaired, inconsistent style of temporal information processing
(13) .
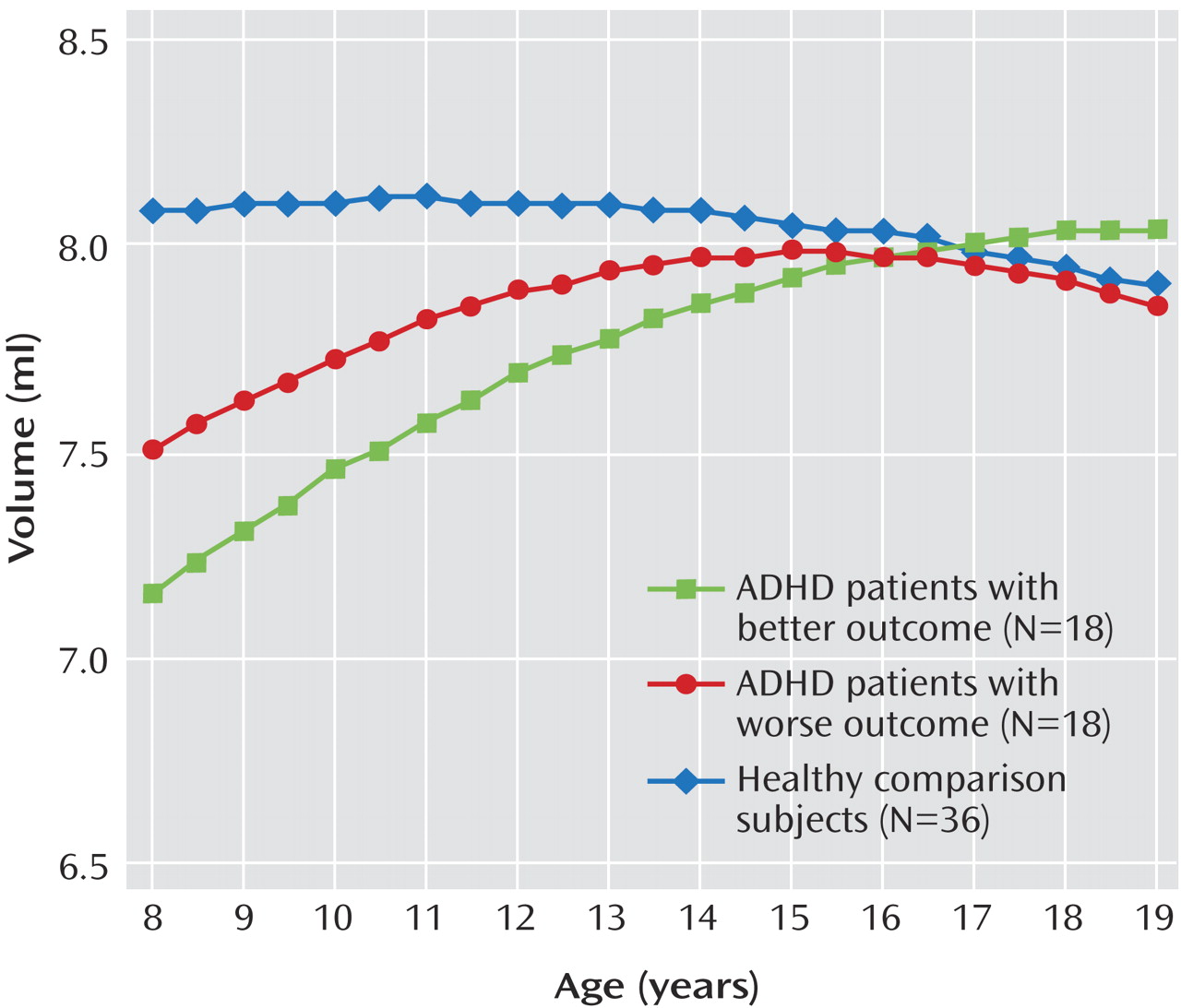
In our study, participants with ADHD in the worse clinical outcome group showed a progressively smaller total cerebellar volume over time, attributable mainly to the deviant trajectory of the inferior-posterior hemispheres. Lesion and functional imaging studies converge to show the importance of the lateral cerebellar hemispheres in executive function
(10,
45), spatial cognition, linguistic processing
(32), and verbal working memory
(31,
46,
47) . Meta-analysis has confirmed moderate deficits in executive functions in ADHD (particularly of response inhibition, set shifting, and working memory)
(48), which are likely to represent one of possibly multiple pathways to the cardinal symptoms of the disorder
(13) . For example, working memory deficits lead to lapses in the maintenance of task-relevant information “online” and could underlie some symptoms of inattention. Additionally, deficits in the cerebellar components of motor control and response inhibition may contribute to hyperactivity. Future work can assess whether the progressively deviant structural trajectory in those with worse clinical outcome in ADHD is accompanied by a pattern of progressive cognitive deficits that contribute to the persistence of symptoms.
As the study participants with ADHD moved into adolescence, the volume of the left anterior hemisphere converged to that of the comparison group. This volumetric normalization occurred in a region that some lesion studies have linked specifically with time perception and precise motor timing
(9) . Recent high-resolution functional imaging studies with healthy subjects have demonstrated marked activation of the anterior and superior hemispheres (lobules V–VI) during the perception and motor performance of temporal sequences
(49) and implicated the anterior and superior cerebellum in visual and memory search
(50) . In adults with persistent ADHD, an anomalous decrease in activation of the superior and anterior hemispheres during working memory tasks directly implicates this region in one of the deficits associated with ADHD
(51) . It is possible that the structural normalization we report in our better outcome group is associated with improvements in these and related cognitive functions, representing a possible plastic compensatory response.
No firm conclusions can be drawn about etiological factors in this descriptive study. Structural features of the cerebellum are highly heritable (with an additive genetic value of 0.49)—although less so than those of other lobes—and thus genetic factors are likely to be important
(52) . It is also possible that the different trajectories within ADHD are determined by polymorphisms in monoaminergic neurotransmitter genes that are expressed in the cerebellum, such as polymorphisms of the dopamine D
4 receptor gene, which have been linked with clinical outcome
(53) . The rates of oppositional defiant disorder and anxiety disorders were higher in the worse outcome group, which raises the possibility that these comorbid disorders partly account for the deviant developmental pathways. This is of particular interest given recent suggestions that the presence of conduct disorder and possibly oppositional defiant disorder defines a phenotypic subtype of ADHD
(54) . However, in all our study subjects at baseline, the clinical picture was dominated by ADHD, and the comorbid conditions were not the focus of treatment. This question will be clarified in future studies with an expanded sample that includes a larger proportion of children with a worse outcome and no comorbid disorders.
Limitations of this study include problems inherent to use of a manual region-of-interest method of measuring the vermis, which is reflected in the ICCs. However, all interrater ICCs were within the range considered good, and all intrarater ICCs were excellent (greater than 0.8)
(55) . Our sample consisted almost entirely of patients with combined-type ADHD, which limits the examination of subtypes of ADHD. While we did not find any statistical difference in whole cerebellar size in children with ADHD, the effect size reflecting the volumetric reduction in ADHD is in line with previous reports, which suggests that the study lacked the power to detect a statistically significant difference. However, by restricting our analysis to children for whom three or more scans were available, we gained the perspective of longitudinal growth trajectories, which refine and extend the volumetric differences noted in previous studies. A large proportion of our subjects were exposed to stimulant medication both prior to the first assessment and during the follow-up period, which complicates the interpretation of our findings because stimulants may have neurotrophic effects
(2) . However, because the two ADHD groups had similar histories of exposure to stimulants at baseline, medication alone is unlikely to account for the differential trajectories linked with outcome. It is more difficult to evaluate systematically the possible trophic effects of other medications given the rarity of use in our sample.
In summary, we found a fixed, nonprogressive volume reduction in the superior cerebellar vermis of children with ADHD, which may prove to be a useful endophenotype in future genetic studies. In contrast, cerebellar hemispheres exhibit a trajectory of developmental growth that is state specific and may represent a potential target for clinical or pharmacological intervention in ADHD.