Obesity (body mass index >30), has increased significantly over the past 30 years (approximately 50% per decade)
(1), afflicting 32.2% of adults in the United States
(2) . Obesity increases risk for cardiovascular disease, diabetes, cancer, and other diseases, resulting in annual health care costs conservatively estimated for the United States at $70 to $100 billion a year
(3) as well as reductions in life expectancy by 5 to 20 years
(4) . These facts highlight the urgent need to develop strategies to prevent and treat those afflicted.
Although there have been major scientific advances in the treatment of the medical complications of obesity (i.e., diabetes, hypertension hypercholesterolemia), the morbidity from this disorder is hampered by the failure of interventions to sustain weight loss. Standard interventions based on promoting lifestyle changes to decrease excessive food consumption (dieting) and increased physical activity (exercise) are effective and can normalize weight if followed rigorously, but unfortunately they are incredibly difficult to sustain. The discrepancy between the successes of the metabolic treatments of consequences of obesity and the failures of behavioral treatments to prevent or reverse obesity highlight the fact that this condition is not only a metabolic disorder but also a brain disorder. Consideration of the mental component of obesity should be a key target in the treatment of obesity to facilitate compliance and minimize relapse. Here, we propose that some forms of obesity are driven by an excessive motivational drive for food and should be included as a mental disorder in DSM-V.
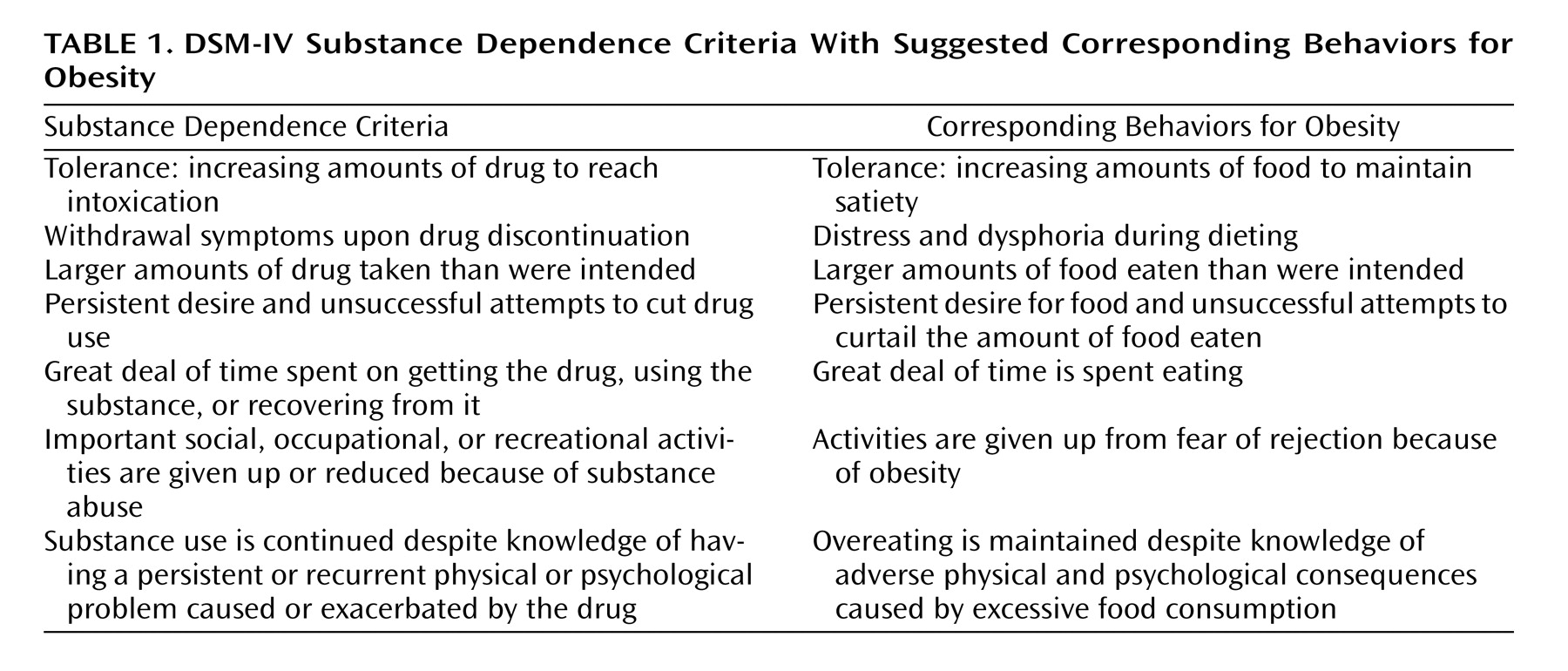
DSM-IV recognizes eating disorders such as anorexia and bulimia as mental disorders with severe impairments and serious adverse outcomes but does not recognize obesity despite its devastating medical and psychological consequences. Obesity is characterized by compulsive consumption of food and the inability to restrain from eating despite the desire to do so. These symptoms are remarkably parallel to those described in DSM-IV for substance abuse and drug dependence (
Table 1 ), which has led some to suggest that obesity may be considered a “food addiction”
(5) .
There are multiple mechanisms contributing to the vulnerability to obesity, including genetic, developmental, and environmental factors that are likely to interact in diverse ways among individuals to produce the behavioral phenotype of overeating
(6) . The “thrifty genotype” hypothesis suggests that evolution shaped the circuits involved in how our bodies store food as well as the circuits involved in the procurement of food in our ancestors when food was scarce. In current environments, where for the most part food is widely available and diverse, these circuits can lead to food overconsumption. The “developmental origin hypothesis” suggests that calorie content as well as exposure to certain nutrients during pregnancy modify how the body and brain develop in anticipation of future environments with similar nutrient characteristics.
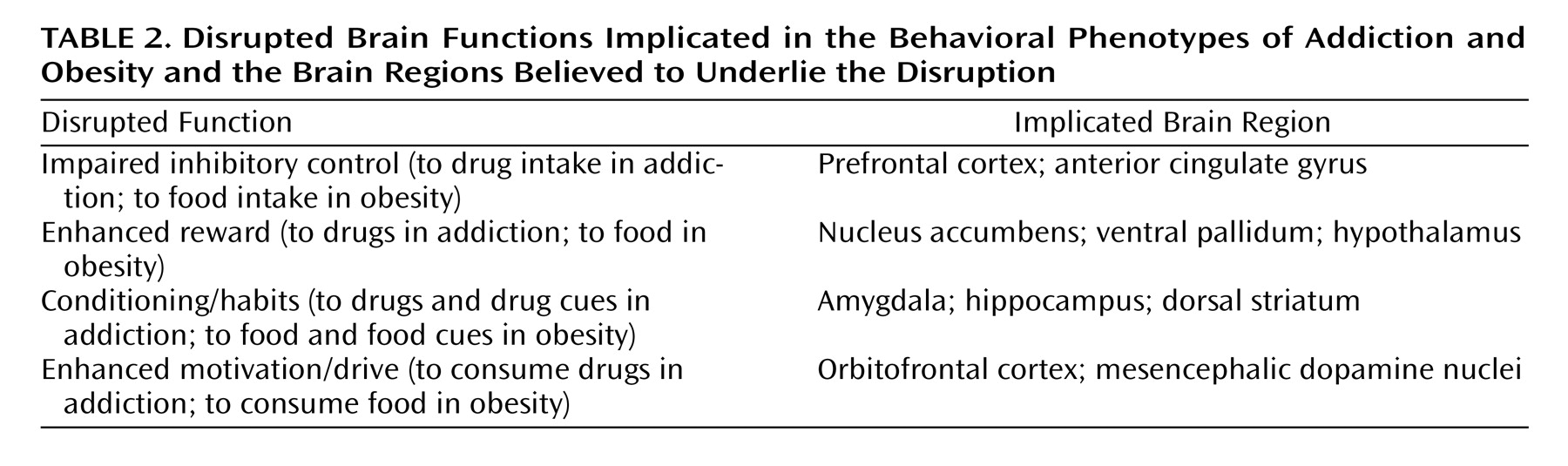
What brain circuits are associated with obesity? The hypothalamus is recognized as the main brain region that controls the regulatory signals for food consumption. The genetic products that modulate hypothalamic activity (i.e., leptin, ghrelin, insulin) are also expressed in limbic brain regions involved with reward, motivation, learning, emotion, and stress responses that are likely to modulate food consumption
(7) . In vulnerable individuals (because of genetic or developmental factors), how do these brain circuits become disrupted to produce compulsive food consumption? As shown in
Table 2, we postulate that the underlying brain mechanisms are similar to those that ultimately result in the compulsive drug consumption in addiction
(8) . Both food consumption and drug use are driven by their rewarding properties, which have been linked to increases in dopaminergic activity in brain reward circuits, but they do this in different ways
(9) . Food activates brain reward circuitry via palatability (mediated in part by endogenous opioids and cannabinoids) and via increases in peptides that modulate dopamine activity (i.e., insulin, leptin)
(10), whereas drugs activate this same circuitry directly through their pharmacological effects (mediated by their direct effects on dopamine cells or by their effects on neurotransmitters that modulate dopamine cells such as opioids, nicotine, GABA, and cannabinoids)
(11) . Repeated supraphysiological dopamine stimulation from chronic drug use is believed to induce plastic changes in brain (i.e., glutamatergic cortico-striatal pathways) that result in poor inhibitory control over drug consumption and compulsive drug intake
(12) . In parallel, dopaminergic stimulation facilitates conditioning to drugs and drug-associated stimuli as well as learned habits that then drive the behavior to take drugs when exposed to stimuli associated with drugs. Similarly, repeated exposure to certain foods (particularly those with a high fat and sugar content) in vulnerable individuals can also result in compulsive food consumption, poor food intake control, conditioning to food stimuli, and, over time, massive weight gain. It is not surprising that there is significant overlap in the medications that have been shown to interfere with drug and food consumption in animal models of drug abuse and obesity respectively (i.e., cannabinoid antagonists, baclofen, GABA agonists, and CRF antagonists) and in the behavioral interventions that are frequently used in the treatment of both conditions (incentive motivation, cognitive behavior therapy, and 12-step programs). Stimulants such as cocaine and methamphetamine can suppress appetite perhaps by satiating the reward system, but they often lead to abuse and to return of overeating when tolerance develops or they are stopped. In contrast, partial blockade of the reward system by antipsychotics (dopamine D
2 receptor antagonists) can result in overeating and can increase the risk for obesity.
The increasing prevalence and impact of obesity in our society and the urgent need to develop better therapeutic interventions that help mitigate the pathologically intense drive for food consumption are clear. We have an opportunity in DSM-V to recognize a component of obesity as a mental disorder. Because of the complex ideologies of obesity it will be important to consider guidelines of which of these deserve to be classified as a mental disorder and which do not. This would facilitate the treatment of obesity not just as a metabolic disorder but also, when appropriate, as a mental disorder.