The vascular depression hypothesis proposes that late-life depression is associated with vascular disease
(1,
2). This hypothesis is based on strong associations between depression and vascular disease and MRI findings in depressed subjects of elevated rates of deep white matter hyperintensities, which may have a vascular origin
(3). There is currently no direct neuropathological evidence supporting this proposition. Intercellular adhesion molecule-1 (ICAM-1) is a vascular marker of inflammation, and its expression is increased by ischemia
(4). This study investigated whether ischemic changes occur in depression by examining the expression of ICAM-1 in the dorsolateral prefrontal cortex. We chose this brain region because positron emission tomography studies have demonstrated hypometabolism in this area in depression
(5,
6). Our hypothesis was that expression of ICAM-1 in the dorsolateral prefrontal cortex in patients with late-life depression would be greater than that in comparison subjects without depression.
Method
Full approval was granted for this postmortem study by the human subjects ethics committee at our institution, and consent for postmortem examination was obtained from the next of kin. We examined tissue from 20 subjects with depression (18 were depressed at death) and 20 nondepressed comparison subjects. The depressed subjects were 60 years old or older at the time of death and had suffered at least one well-documented episode of DSM-IV major depression but had never met DSM-IV criteria for dementia or other major psychiatric disorder. Comparison subjects met the same criteria except they had never suffered a depressive episode. The cause of death was cardiac arrest for eight comparison subjects and seven patients, pneumonia for three comparison subjects and seven patients, other respiratory disease for two comparison subjects and two patients, carcinoma for four comparison subjects and one patient, suicide for two patients, and other for three comparison subjects (hematemesis, mesenteric infarction, liver failure) and one patient (renal failure).
The groups were comparable in vascular risk factors: myocardial infarction for five comparison subjects and three patients, hypertension for four comparison subjects and seven patients, diabetes for two comparison subjects, and smoking for seven comparison subjects and seven patients. All depressed subjects had undergone extensive assessment by experienced psychiatrists. All subjects had a postmortem assessment and were excluded if they met neuropathological criteria for any neurological disorder or any known cause of dementia, such as Alzheimer’s disease.
After death, the right hemisphere was fixed in 10% buffered formalin. Full coronal face blocks were obtained from the prefrontal cortex, including the dorsolateral prefrontal cortex (Brodmann’s areas 9, 10, and 46), and from the occipital cortex (Brodmann’s areas 19 and 39). The latter was a comparison area to see whether any differences found between subjects with and without depression showed specificity for the dorsolateral prefrontal cortex. Blocks were embedded in paraffin wax, 10-mm sections were prepared, and indirect immunocytochemistry was carried out on slides from each area with appropriate secondary antibodies, avidin-biotinylated horseradish peroxidase complex (Vector Laboratories, Ltd., Peterborough, U.K.), and diaminobenzidine as Chromagen. Collagen IV, a marker of the density of the microvascular tree, was measured to determine whether any differences in ICAM-1 reflected differences here rather than in ICAM-1 expression in the vessels
(7). Primary antibodies were a polyclonal antibody to ICAM-1 (R&D Systems, Abingdon, U.K.) (1:500 dilution) and a monoclonal antibody to collagen IV (Sigma, St. Louis) (1:500 dilution).
Fifteen images were captured from both gray and white matter in each cortical area using an objective lens with a magnification of 10 on a Zeiss Axioplan 2 light microscope coupled to a three-chip CCD true-color video camera (JVC KY F55B). They were analyzed by two independent raters blind to diagnosis on a monitor using a standard software program (Image Pro Plus, version 4.0; Media Cybernetics, Silver Spring, Md.). This involved measuring the percentage area of diaminobenzidine staining in each image and calculating a mean score in each area for gray and white matter.
Statistical comparisons of the two groups (unpaired t tests, analyses of variance [ANOVAs], and Pearson correlations) were made by using SPSS software (version 7.5) (Chicago). Interrater and intrarater reliability ratings were calculated by using the intraclass correlation coefficient and the coefficient of variation, respectively.
Results
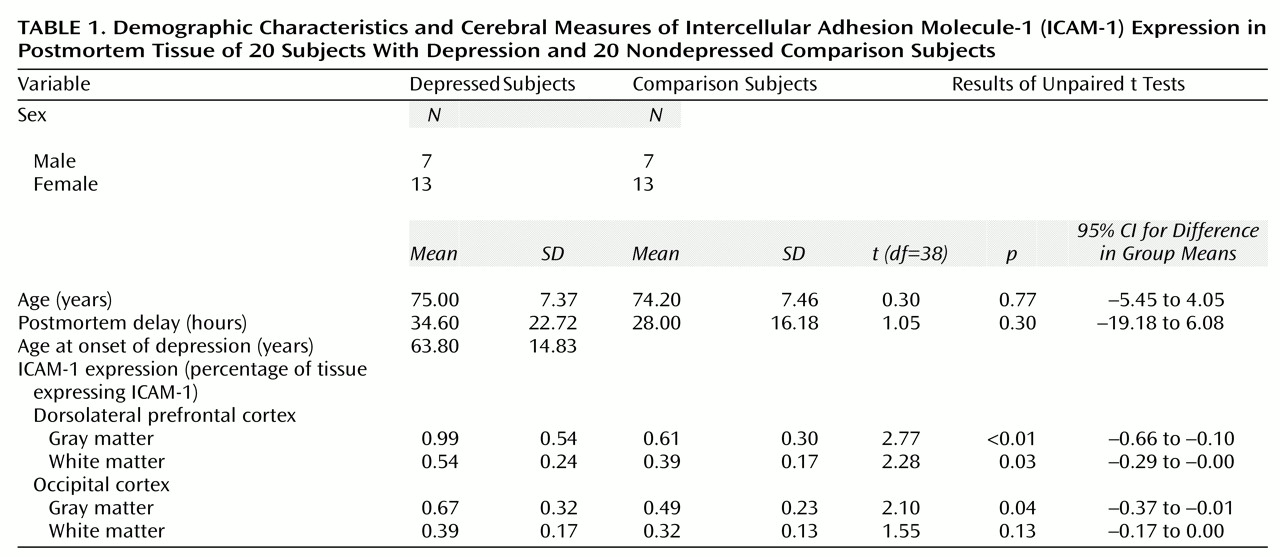
Subjects’ characteristics and ICAM-1 results, showing significantly elevated ICAM-1 in both gray and white matter in the dorsolateral prefrontal cortex in depression, are shown in
Table 1. Coefficients of variation for rater 1 were 4.2% (collagen IV) and 3.9% (ICAM-1) and for rater 2 were 7.5% (collagen IV) and 9.8% (ICAM-1). The intraclass correlation coefficient was 0.97. The collagen IV scores showed no significant differences in either the dorsolateral prefrontal cortex (t=1.43, df=38, p>0.16) or the occipital cortex (t=0.85, df=38, p>0.40). Pearson correlations of the ICAM-1 scores showed no relationship with postmortem delay (r=0.08, N=40, p>0.62) or age (r=0.14, N=40, p>0.38). ANOVA showed no significant contribution of ever having been treated for hypertension to the ICAM-1 scores (F=0.95, df=2, 35, p>0.40) and no significant interaction between diagnosis and hypertension (F=0.10, df=1, 35, p>0.75). There was no evidence of a treatment effect: t tests revealed no differences in any area between depressed subjects who were (N=12) or were not (N=8) taking antidepressant medication at death (t=0.56, df=18, p>0.58) and those who had (N=11) and those who had not (N=9) ever received ECT (t=1.06, df=18, p>0.30).
Discussion
There was greater ICAM-1 expression in the dorsolateral prefrontal cortex in depressed subjects than in nondepressed subjects, independent of potential confounders such as age or treatment. Elevated ICAM-1 expression was more marked in the gray matter (62% elevation) than in the white matter (38% elevation). It also showed some specificity for the dorsolateral prefrontal cortex because there were no differences between depressed and nondepressed subjects in the white matter and only a modest difference in the gray matter in the occipital cortex.
This up-regulation of ICAM-1 is consistent with the hypothesis there are ischemia-induced inflammatory changes in the dorsolateral prefrontal cortex in late-life depression, provides direct support for the vascular depression hypothesis
(1,
2), and, to our knowledge, is the first neuropathological evidence of a vascular abnormality in late-life depression.
The lack of difference between depressed and nondepressed groups in collagen IV showed that the ICAM-1 results reflect true differences in expression of the molecule between groups rather than differences in the density of the vascular tree. Neuropathological assessment excluded Alzheimer’s disease, other causes of dementia, and stroke as possible explanations for our results. Hypertension did not explain the up-regulation of ICAM-1, which suggests that a combination of vascular processes may be involved. Varying contributions from atheromatous disease, microvessel disease, and hemodynamic changes in different patients might together produce the ischemia-induced inflammation. We cannot exclude the possibility, however, that depression itself causes changes in ICAM-1 expression and that, if such a relationship exists, it could be related to hypothalamic-pituitary-adrenal (HPA) axis activation and cytokines. In their review, Connor and Leonard
(8) concluded that immunological activation, including increases in the circulating cytokines IL-1 and γ-IF, does occur in some depressed patients. These cytokines stimulate the HPA axis (8) as well as inducing ICAM-1, thereby raising the tantalizing possibility of a relationship among depression, ICAM-1, cytokines, and the HPA axis.
Although further investigation is required into the causes of up-regulation of ICAM-1, our findings have important implications for understanding etiological mechanisms in late-life depression and for altering future management.