The neurochemistry of depersonalization is poorly understood and does not provide clear indication of a neuroanatomical center. Depersonalization can be induced in subjects not suffering from the disorder by means of a pharmacological challenge with tetrahydrocannabinol (THC)
(3) or the partial serotonin agonist m-CPP
(4). Attempts at localizing depersonalization, although not in depersonalization disorder per se
(5–
12), have yielded contradictory results regarding activation, laterality, and regional involvement. Half a century ago, Penfield and Rasmussen
(13) noted “illusions of unfamiliarity, strangeness and remoteness,” the descriptions of which reflect typical depersonalization experiences. They claimed that these perceptual illusions could be produced by stimulation of the cortex only in the temporal region, perhaps extending somewhat into the occipital cortex (p. 173). In “G.A.,” “queer sensations of not being present and floating away” were produced by stimulation of the superior temporal gyrus. “D.A.” experienced illusions of being “far off and out of this world,” produced by stimulation of the middle temporal gyrus. It is of interest that Penfield and Rasmussen postulated that depersonalization states involve an “alteration in the usual mechanism of comparison of immediate sensory perception with memory records.”
In addition, the subjective symptoms encountered in depersonalization disorder are overwhelmingly perceptual in nature, involving primarily the visual and somatosensory modalities. Therefore, examination of sensory activity in the parietooccipital cortex would be of great interest.
In summary then, the goal of the present study was to localize brain function abnormalities associated with depersonalization disorder, with a particular focus on the temporal lobe hypothesis, the frontolimbic disconnection hypothesis, and the function of the sensory cortical network.
Results
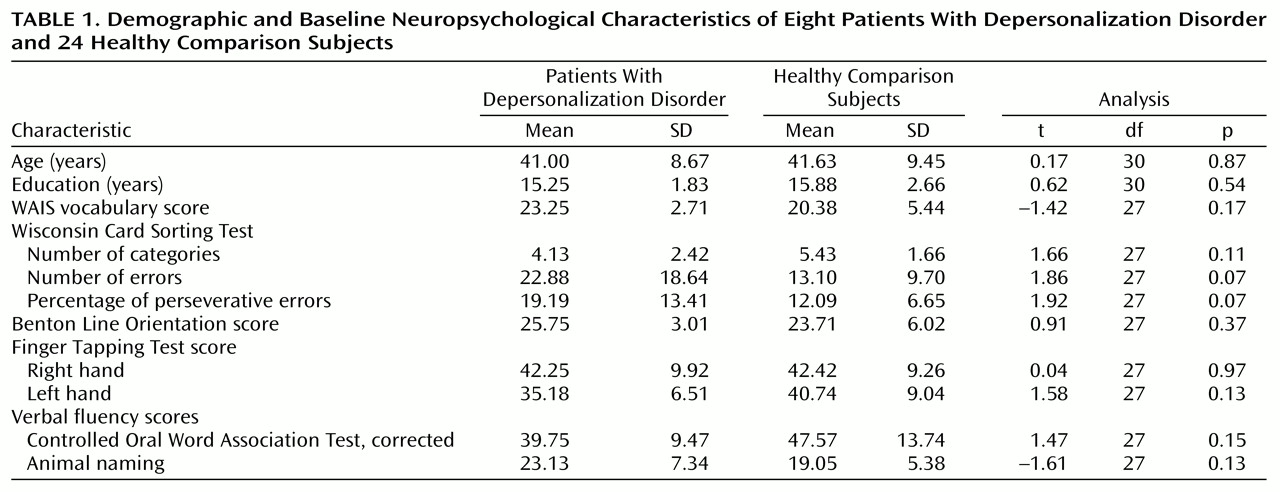
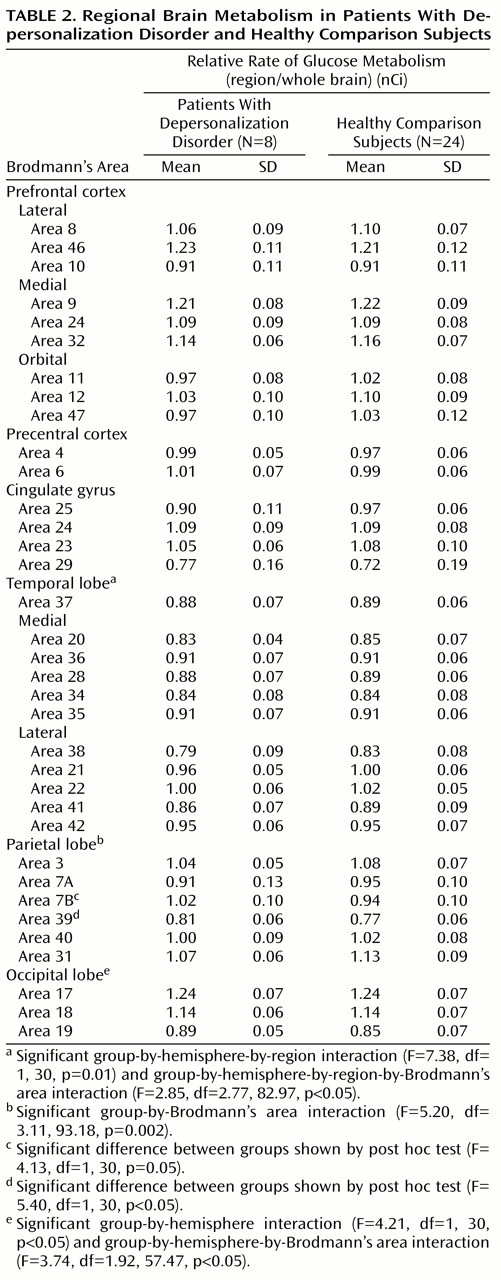
Each analysis was examined for main effect of group and for all higher-order interaction effects. Results are summarized in
Table 2, and findings were as follows. No statistically significant differences were found for the prefrontal cortex, precentral cortex, or cingulate cortex.
The temporal lobe was subdivided into medial and lateral regions, each with five Brodmann’s areas. There was a significant group-by-hemisphere-by-region interaction and a significant group-by-hemisphere-by-region-by-Brodmann’s area interaction (
Table 2). Post hoc comparisons revealed that the depersonalization disorder group had significantly lower metabolic rates in area 22 of the right superior temporal gyrus (subjects with depersonalization disorder: mean=1.06, SD=0.06; healthy comparison subjects: mean=1.11, SD=0.06; t=2.24, df=30, p<0.05) and in area 21 of the middle temporal gyrus (subjects with depersonalization disorder: mean=0.99, SD=0.05; healthy comparison subjects: mean=1.04, SD=0.05; t=2.61, df=30, p=0.01).
The parietal lobe revealed a significant group-by-Brodmann’s area interaction (
Table 2). Post hoc between-group comparisons by Brodmann’s area revealed significantly higher metabolic rates in the depersonalization disorder group for areas 7B and 39 (
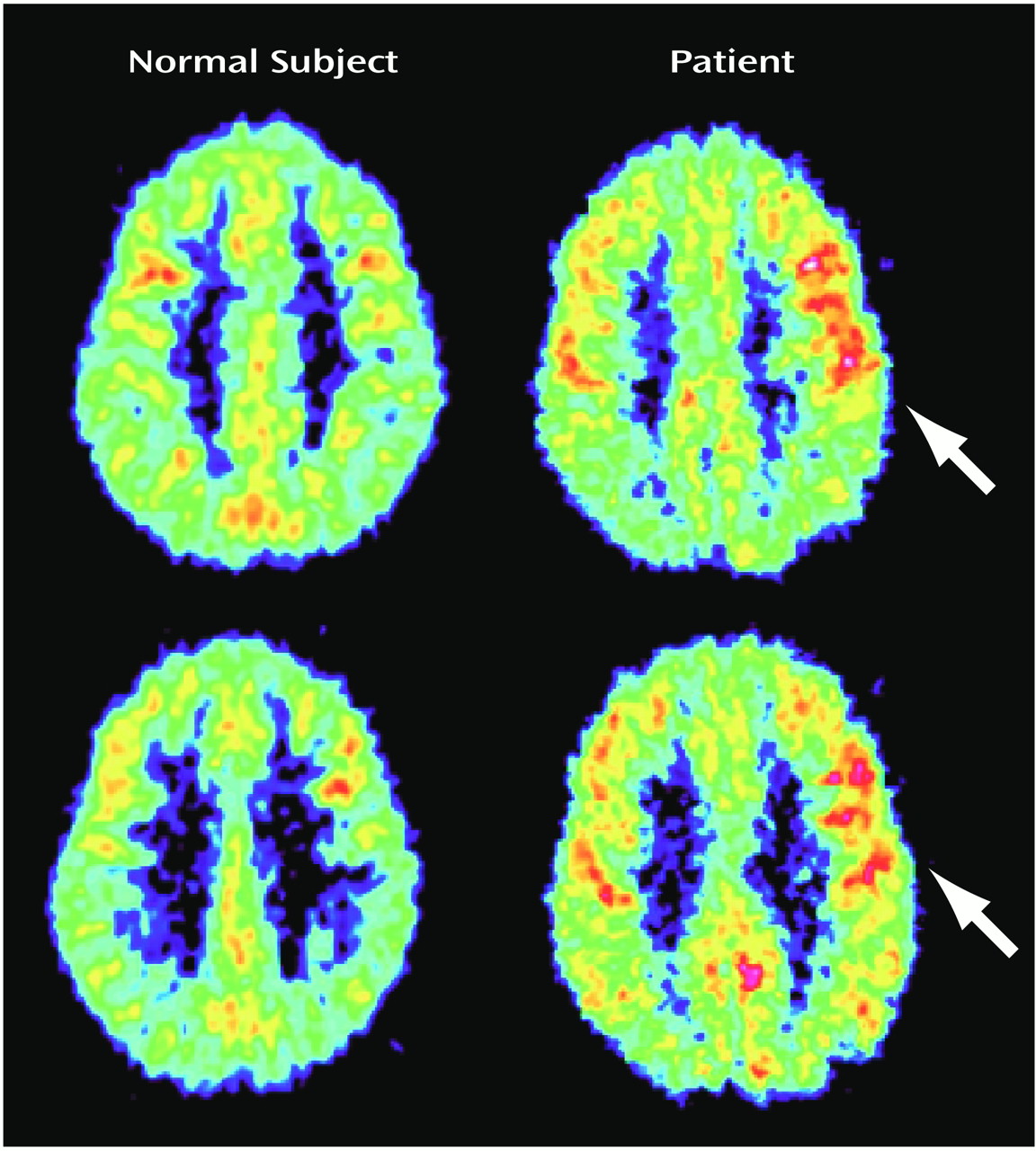
Figure 1 and
Table 2). There was a significant positive correlation of relative glucose metabolic rate with total Dissociative Experiences Scale score and Dissociative Experiences Scale depersonalization score for area 7B (r=0.84, df=6, p<0.01, and r=0.74, df=6, p<0.05, respectively).
The occipital lobe revealed a significant group-by-hemisphere interaction effect and a significant group-by-hemisphere-by-Brodmann’s area interaction (
Table 2). Post hoc comparisons revealed a significant group-by-Brodmann’s area interaction for the left occipital lobe (F=3.28, df=1.62, 48.54, p=0.05); left area 19 was significantly more active in the depersonalization disorder group (subjects with depersonalization disorder: mean=0.90, SD=0.05; healthy comparison subjects: mean=0.83, SD=0.07; t=2.37, df=30, p<0.05).
Subsequently, an analysis of the whole brain sensory association cortex was conducted, organized into four functional associative regions consisting of two Brodmann’s areas each (temporal areas 22 and 37, occipital areas 18 and 19, parietal multimodal areas 39 and 40, and parietal somatosensory areas 7B and 23). There was a significant group-by-hemisphere interaction (F=5.49, df=1, 30, p<0.05), with a tendency for depersonalization subjects to have more active association areas in the left hemisphere. There was also a significant group-by-associative-region-by-Brodmann’s area interaction (F=4.78, df=1.78, 53.26, p=0.01), demonstrating that depersonalization disorder subjects had an extensive pattern of altered metabolic activity in the major sensory association areas across brain lobes.
Discussion
The main findings of this first (to our knowledge) functional imaging study of depersonalization disorder point to metabolic abnormalities primarily in the posterior cortex. Subjects with depersonalization disorder differed in relative glucose metabolic rate from comparison subjects in portions of the sensory cortex in the temporal, parietal, and occipital lobes. These specifically included right temporal area 22 (auditory association area), parietal areas 7B (somatosensory association area) and 39 (multimodal association area), and left occipital area 19 (visual association area). Depersonalization disorder subjects were characterized by greater activity than comparison subjects in all these areas, with the exception of area 22, where activity was lower. Analyses of the relative glucose metabolic rate in whole brain sensory cortex confirmed an extensive pattern of significant between-group differences.
These data do not support the primacy of temporal lobe phenomena in depersonalization, described in the introduction
(5,
6,
13,
14), but rather, they implicate more extensive associational brain networks, given the prominent occipital and parietal findings. The perceptual alterations that are hallmark symptoms of depersonalization prmarily involve two sensory modalities, visual and somatosensory, although auditory disturbances can also be described. There is a hierarchy of sensory processing in the brain, from primary sensory areas to unimodal and then polymodal association areas and finally to the prefrontal cortex
(27). Unimodal association areas showed more activity in depersonalization disorder subjects, both in occipital area 19 of the prestriate visual cortex and parietal area 7B, which is believed to be central to high-order integration within the somatosensory system
(28). Dissociation and depersonalization scores showed a strong positive correlation with area 7B activity. Multimodal sensory integration occurs in the region of the parietal-temporal-occipital junction or the inferior parietal lobule
(27,
29). Area 39, which corresponds to the angular gyrus and is implicated in somatosensory-visual-auditory integration, was again more active in depersonalization disorder subjects.
It is, however, possible that the differences in metabolic activity identified in this study were somehow related to the task performance itself, although the absence of performance differences on the verbal memory task administered during FDG uptake renders this less likely. In addition, since greater prefrontal activation was not seen in depersonalization, a stronger effort by the depersonalization disorder subjects to achieve similar task scores appears a less likely explanation
(30). It is possible that the greater activation observed in depersonalization disorder reflects compensatory adjustment
(31), as processing is shifted from right temporal lobe areas 22 and 21 to parietooccipital regions. Such a shifting of cognitive function has been observed with aging
(25,
32,
33).
The altered subjective experience of the relationship of the self to the physical body is a uniquely fascinating aspect of depersonalization disorder, as elaborately described by Schilder
(34). Individuals with depersonalization disorder commonly feel detached from their physical selves. Disturbances of body schema are primarily based at the parietal-occipital junction around the angular gyrus, where visual and somatosensory information is integrated to provide an intact well-integrated body image
(35), which again includes area 39. Indeed, Ackner
(36) described inferior parietal and angular tumors manifesting with depersonalization. Maximum overlap of structural lesions in neurology patients suffering from neglect has been found to concentrate in the right inferior parietal lobule
(37). We speculate that body schema distortions characteristic of depersonalization might be more subtle, functionally based, less neurologically damaged versions of well-known parietal lobe neurological syndromes such as neglect, finger agnosia, and hemidepersonalization. The psychiatric version might be characterized by an “as if” quality to the experience of bodily detachment, whereas in the neurological version, entire body parts or sides are treated as truly absent or not part of the self.
Additional subjective depersonalization experiences might be accounted for by the dysfunctional areas localized in this study. Depth perception is associated with the visual association cortices of areas 18 and 19
(38) and with the parietal association cortex
(39). This might explain the flattened, two-dimensional perspective commonly described in depersonalization. Visuoconstructive abilities such as block constructions and the block design subtest of the WAIS have been localized to the posterior parietal area
(38), and dysfunction here could be associated with the inferior WAIS block design performance in depersonalization
(2). Subjects with depersonalization sometimes describe difficulty evoking visual imagery, which is thought to be mediated by visual association areas 18 and 19 and by higher-order visual cortical centers at the occipital-temporal-parietal junction
(38).
There have been several studies addressing the possible nature and localization of brain dysfunction associated with the symptom of depersonalization
(7–
12), although not in subjects with depersonalization disorder. Depersonalization is a common sequel to traumatic head injury, and it appears more likely to occur after mild injury and with high comorbidity for posttraumatic stress disorder, suggesting that predictable anatomical correlates are unlikely in this population
(7). In a single subject without other psychiatric history, a quantitative EEG of alcohol-induced transient depersonalization revealed generalized slowing attributed to metabolic encephalopathy
(8). In contrast to subjects with panic disorder without depersonalization, subjects with depersonalization during panic attacks show bilateral unresponsiveness and slowing on EEG during normally expected, odor-stimulated temporolimbic activation
(9).
We located three functional imaging studies describing the induction of depersonalization in healthy volunteers. Intravenous infusion of THC in 59 subjects resulted in an increase in global cerebral blow flow, most pronounced in the right hemisphere, frontal lobes, and anterior cingulate, with a relative decrease in subcortical structures
(10). THC-induced depersonalization was significantly positively correlated with right frontal and right cingulate blood flow. The authors proposed that depersonalization may be a state of heightened activation and emotional consciousness resulting from a cingulate-mediated decoupling between cortical and subcortical structures. In a PET study of psilocybin-induced psychosis in seven healthy volunteers, increases in ventral striatum dopamine significantly correlated with depersonalization
(11). However, depersonalization did not appear to be a pure state but rather was associated with significant mood and psychotic-like disturbances. Similarly, in a PET study of amphetamine-induced manic-like states in 10 healthy volunteers, there was a widespread increase in cerebral metabolism that was significant for the anterior cingulate, striatum, and thalamus
(12), but again, mood changes were more prominent than depersonalization.
These studies taken together yield interesting but contradictory results regarding brain changes associated with depersonalization, including laterality versus bilaterality, activation versus slowing, localization versus diffuseness, and the regions possibly implicated, such as the frontal, temporal, anterior cingulate, and subcortical areas. These discrepancies could be due to methodological differences as well as sample heterogeneity, i.e., subjects experiencing depersonalization as a symptom in the context of another condition such as epilepsy, encephalopathy, traumatic brain injury or panic disorder, or normal subjects undergoing chemical induction of depersonalization, among other symptoms, by means of various pharmacological agents. It is unclear whether all of these states are phenotypically equivalent to primary depersonalization disorder.
A theoretically extrapolated neurobiological model of depersonalization was proposed recently by Sierra and Berrios
(15). Its basic premise is bilateral corticolimbic disconnection, with left medial prefrontal activation and reciprocal amygdala inhibition resulting in hypoemotionality and decreased arousal, and right dorsolateral prefrontal activation with reciprocal anterior cingulate inhibition leading to hypervigilance, attentional difficulties, and mind emptiness. Our own data did not demonstrate changes in prefrontal or cingulate activity and thus do not lend support to this model. Some of the studies previously summarized support the model with regard to prefrontal activation and amygdala inhibition
(10), whereas findings of cingulate activation
(10,
12) contradict the model. Sierra and Berrios also suggested that in order to explain how depersonalization can be sensory-modality specific in different patients, the putative disconnection may occur at an earlier stage of emotional processing; such an hypothesis is more in accordance with the sensory cortical findings of this study.
In conclusion, the findings of this first (to our knowledge) functional imaging study of depersonalization disorder suggest abnormalities primarily along sequential hierarchical areas, unimodal and cross-modal, of the visual, somatosensory, and auditory processing pathways, as well as in areas responsible for an integrated body schema. They seem to be in good concordance with the phenomenological conceptualization of depersonalization disorder as the dissociative disorder in which there is a failure to integrate perception with the sense of self, as well as with specific experiences that subjects describe. Further clarification of the role of the limbic system and the amygdala, in particular, is needed, as affective memory connections to past experience could play an important role in making new perceptions feel familiar and real
(40). The limitations of this study include the relatively small study group size, the use of a memory rather than a depersonalization induction task, and the use of relative rather than absolute metabolic rate of glucose. Depersonalization is a relatively rare disorder, but we have increased the study’s statistical power by adding a larger group of age- and sex-matched healthy comparison subjects. The study would have required 44 patients for it to be able to detect a group difference in the dorsolateral prefrontal cortex given the observed effect size, but we did confirm parietal, occipital, and temporal lobe differences that had much larger effect sizes. We considered a variety of cognitive or introspective tasks, but the validity and reliability of memory tasks, task applicability to healthy comparison subjects, and moreover, the possible relationship of memory function to the illusion of unfamiliarity recommended the current task. Last, we examined metabolism relative to whole brain metabolic rate rather than absolute rate, in micromoles per 100 grams per minute. This analysis of ratio numbers allows individual differences in global brain metabolic rates to be removed, a correction made with either ratios or linear covariance in a large majority of current reports. If a small number of small areas had similar values in absolute metabolic rates in both groups while the remaining large areas of the brain had higher metabolic rates in the patient group, misleading lower rates in the small areas could possibly be reported for patients. However, it is well known that sensory task activation tends to be expressed with greater statistical power when correction for global brain variation is removed. Higher test-retest correlations over time have been observed with ratio than with absolute metabolic rate data
(41), as well as with blood flow data from single photon emission computed tomography
(42), suggesting that ratio data are appropriate for examination of regional metabolic trait differences between groups. Furthermore, the nature of the limited and specific behavioral deficits of depersonalization do not suggest a large and diffuse cortical change but rather one focused on limited cortical areas, as noted earlier.