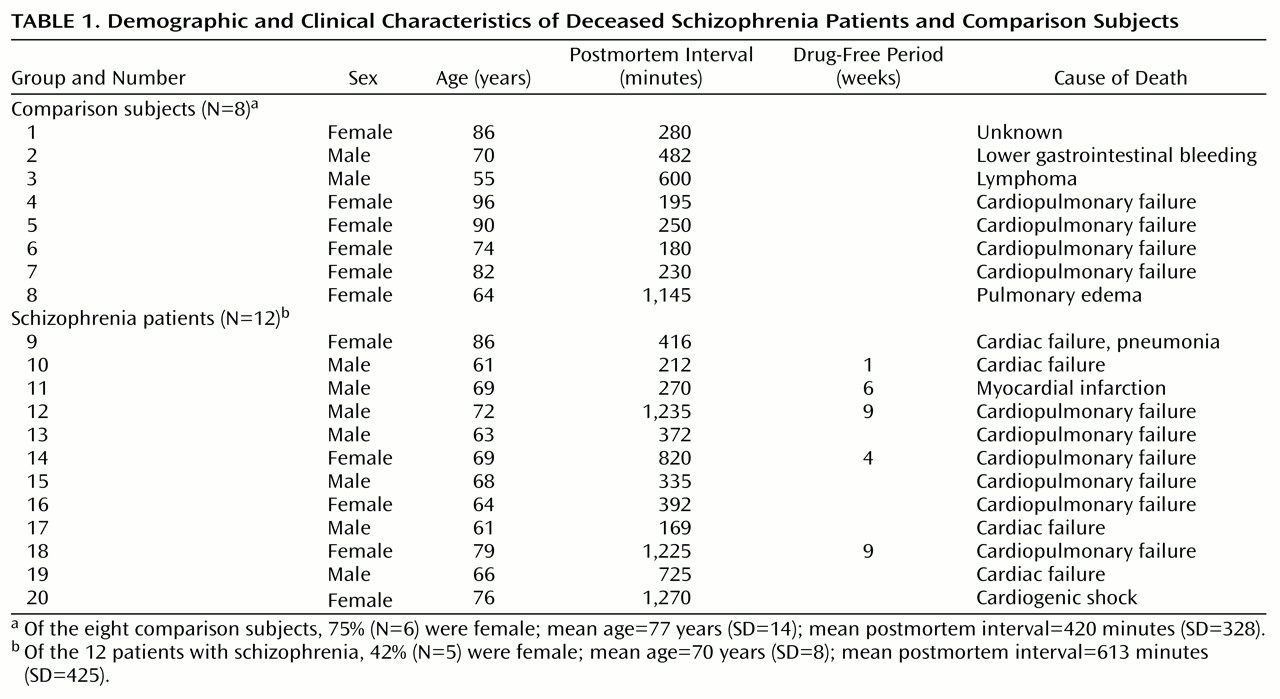
The critical role of the thalamus in sensory processing, and the rich, reciprocal limbic projections with the frontal and cingulate cortex, hippocampus, nucleus accumbens, and amygdala, has led to speculation that this structure may be dysfunctional in schizophrenia
(1). Structural and functional pathology have been detected in the thalamus in schizophrenia, which is consistent with this hypothesis. Lower thalamic cell numbers and volume have been reported in some but not all studies in schizophrenia, in relation to comparison subjects, while lower levels of thalamic metabolism and suggestions of different corticothalamic connectivity have been consistently reported
(2–
13). However, it is currently unclear which neurochemical substrates are associated with these abnormalities. The glutamatergic system represents a likely candidate, both because most thalamic afferents and efferents are glutamatergic
(1,
14,
15) and because pharmacological evidence implicates glutamatergic dysfunction in schizophrenia.
N-Methyl-
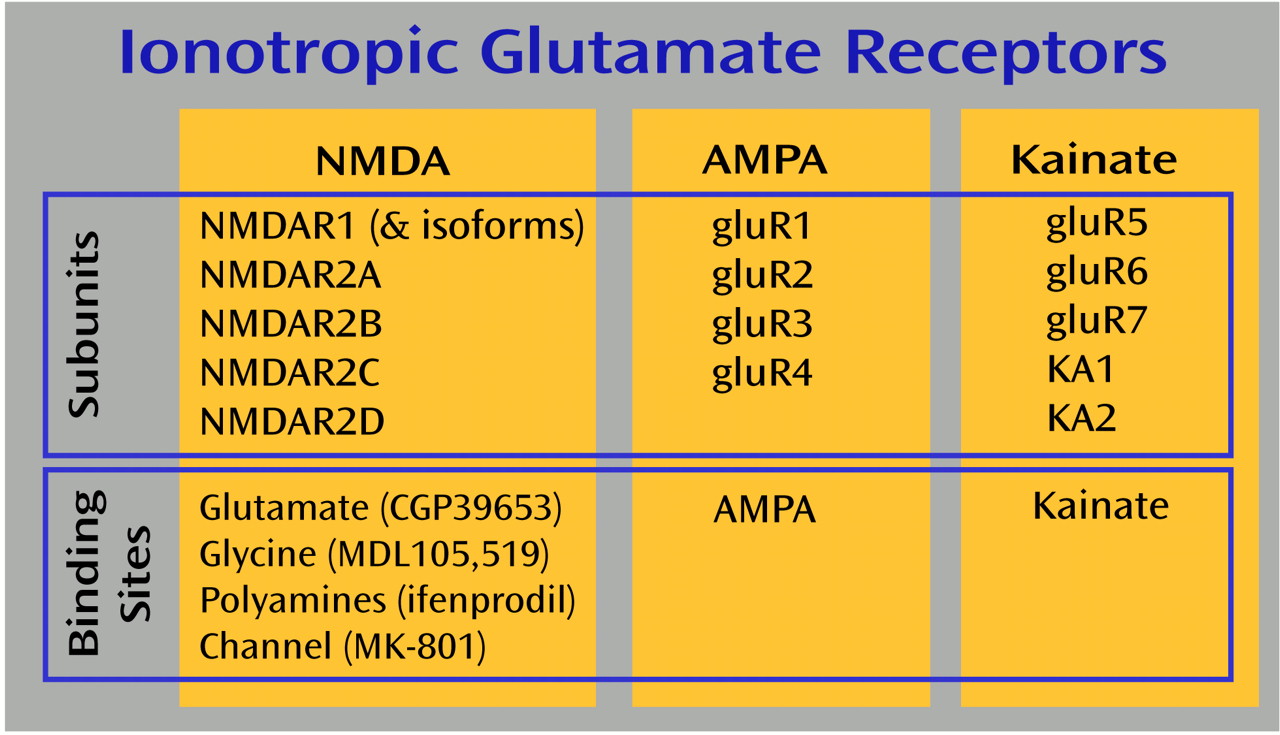
d-aspartate (NMDA), AMPA, kainate, and metabotropic receptors make up the four families of glutamate receptors, and all are expressed in the thalamus
(16,
17). NMDA receptor abnormalities are most often associated with schizophrenia, because the NMDA receptor antagonists phencyclidine (PCP) and ketamine can induce schizophreniform psychosis in normal volunteers and exacerbate psychotic symptoms in schizophrenia patients
(18–
22). Furthermore, adjunct treatment with conventional antipsychotics of agonists and partial agonists of the glycine coagonist site of the NMDA receptor has been reported in some studies to ameliorate negative psychotic symptoms
(23–
28). These data have been interpreted to suggest that NMDA receptor hypoactivity is associated with schizophrenia. However, it is not apparent whether a difference in NMDA receptor activity results from a primary defect in NMDA receptors or from dysfunction in one of the other three glutamate receptor families that may secondarily lead to low levels of NMDA receptor activity. Activation of presynaptic kainate receptors facilitates glutamate release and/or decreases GABA(γ-aminobutyric acid)ergic activity, creating a functional interface with postsynaptic NMDA receptors
(29–
36). Furthermore, the NMDA receptor ion channel is blocked by physiological concentrations of magnesium ions, and partial depolarization of the cell membrane is required to extrude magnesium and allow ion flow though the NMDA receptor channel. Activation of AMPA receptors appears to provide this permissive function, and AMPA receptors are extensively colocalized with NMDA receptors at glutamatergic terminals
(37). Finally, there are pre- and postsynaptic metabotropic receptors that also affect NMDA receptor-mediated neurotransmission
(38–
40). Dysfunction of any of the four glutamate receptors could mimic abnormal NMDA receptor activity.
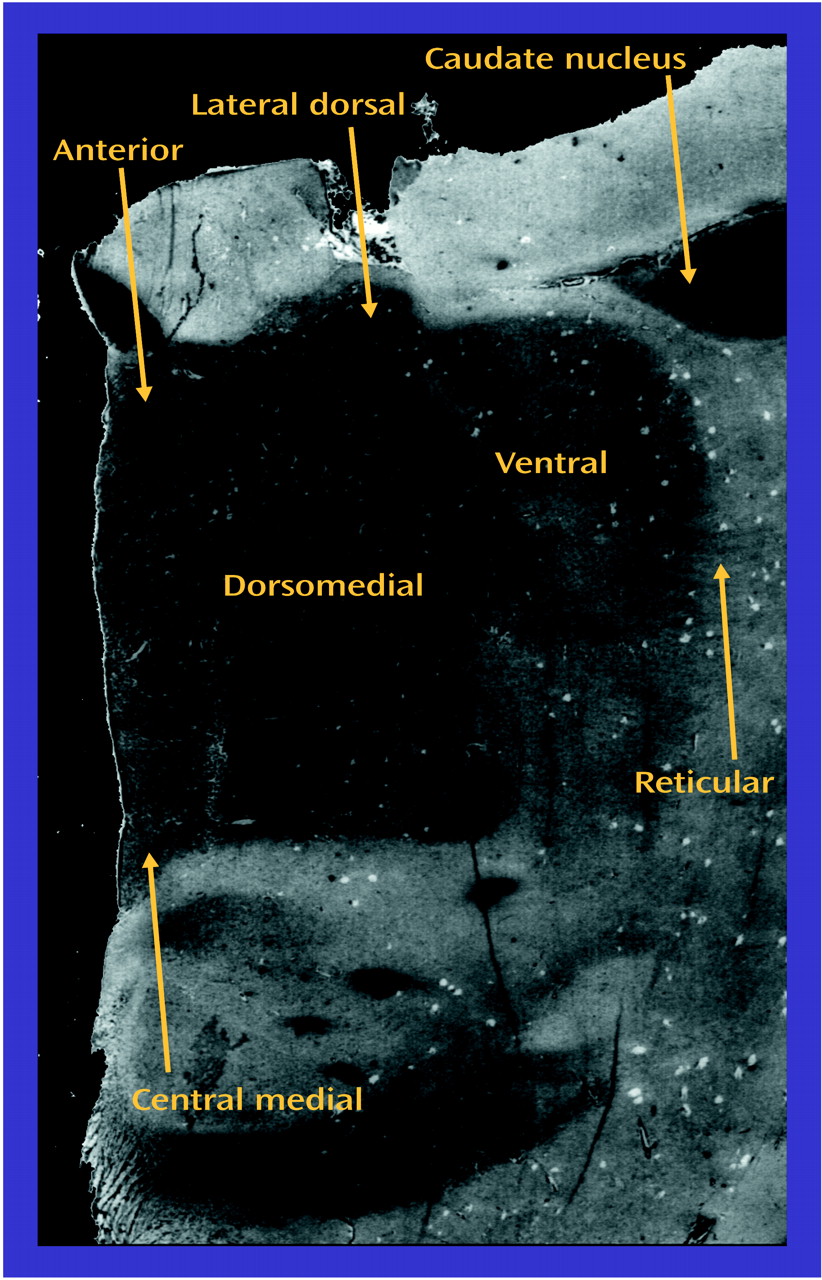
Both thalamic and glutamatergic dysfunction have been separately associated with schizophrenia, but there are few studies examining thalamic glutamate receptor expression in this illness. Therefore, we measured ionotropic glutamate receptor subunit mRNA levels by in situ hybridization, and receptor binding by receptor autoradiography, in discrete thalamic nuclei in patients with schizophrenia and comparison subjects. The topographic organization of the thalamus allowed us to compare glutamate receptor expression in the limbic nuclei (dorsomedial, anterior, laterodorsal, and central medial) with nonlimbic nuclei (reticular and ventral). Our overall hypothesis was that glutamate receptor expression differs in the limbic thalamic nuclei in schizophrenia.
Discussion
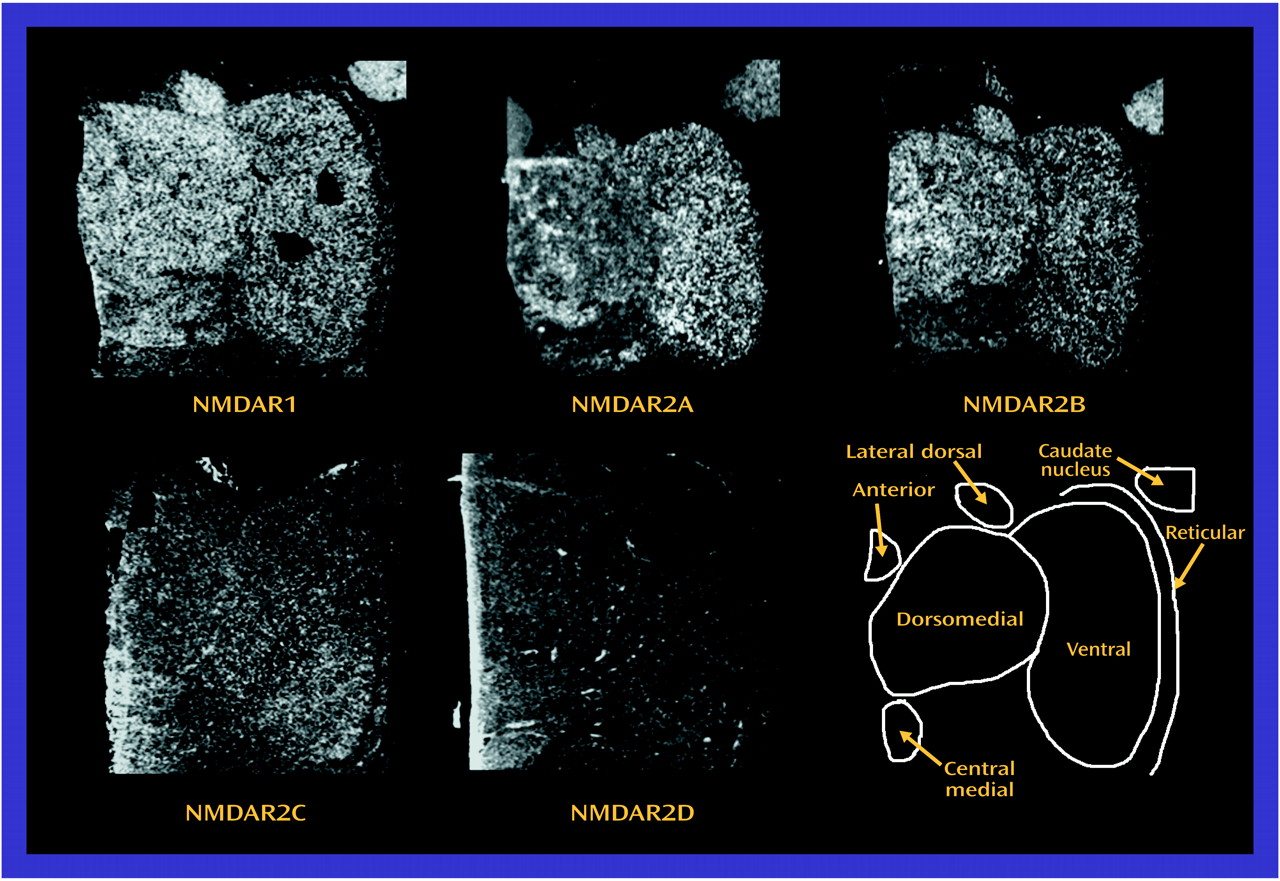
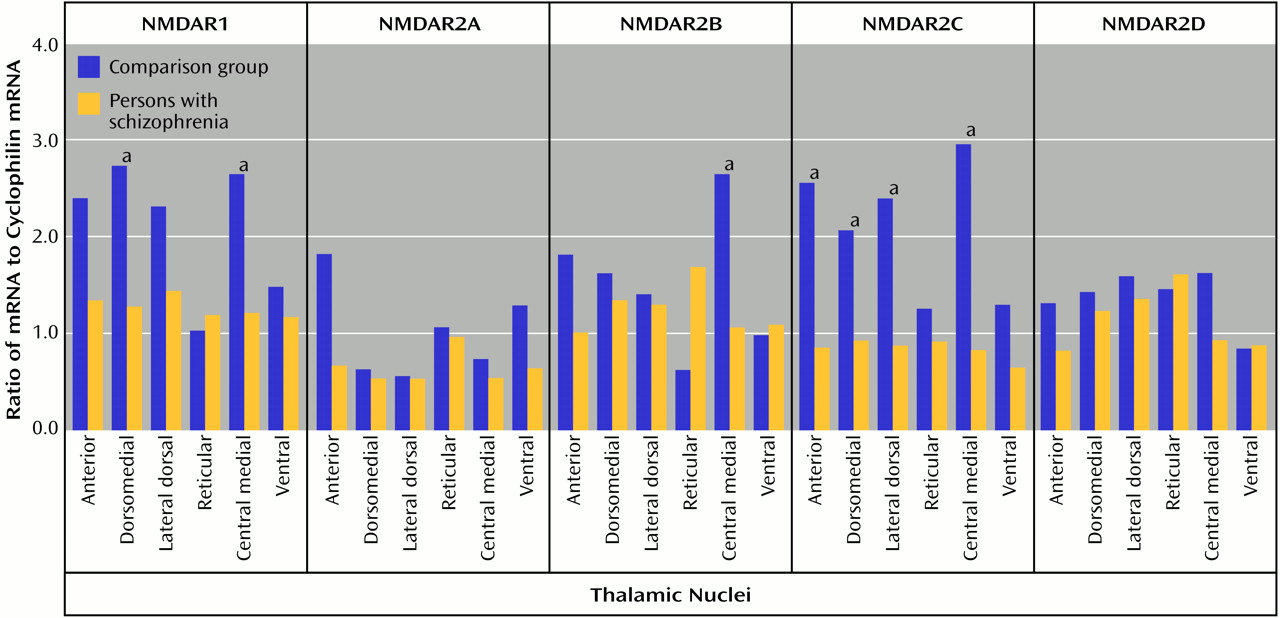
In the present study, significantly lower levels of thalamic glutamate receptor expression were detected in the patients with schizophrenia than in the comparison subjects, primarily involving the NMDA receptor and restricted to limbic nuclei. To our knowledge, this is the first demonstration of different ionotropic glutamate receptor expression found in the thalamus in schizophrenia. The differences in NMDA receptor expression are limited to three of the five subunits and two of the four measured binding sites, while abnormalities of AMPA and kainate receptor expression are limited to certain subunit mRNA levels and do not involve differences in final receptor binding site expression. Therefore, there are subunit and binding site-specific abnormalities in ionotropic glutamate receptor expression in the limbic thalamic nuclei in schizophrenia.
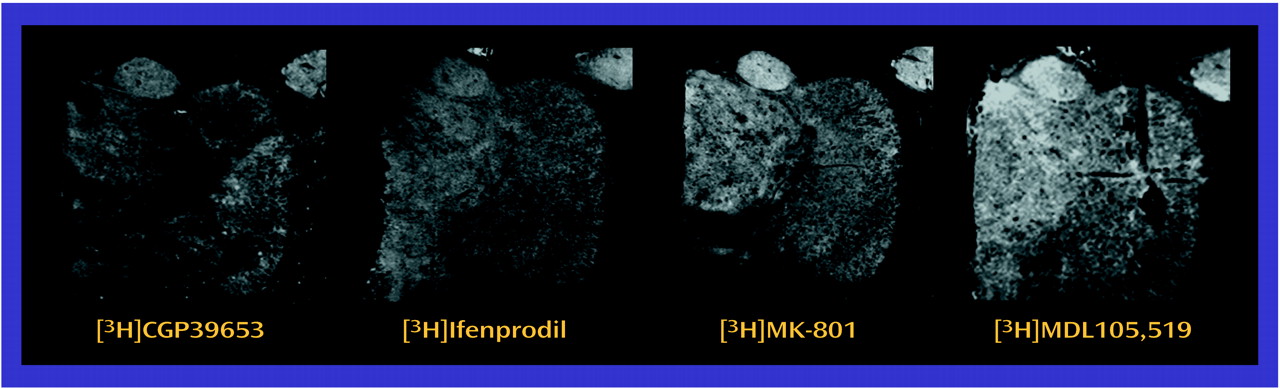
The observed differences in NMDA receptor subunit mRNA levels may reflect differences in NMDA receptor composition in the thalamus. NMDA receptor subunit composition confers unique pharmacological characteristics to assembled receptors. NMDAR1 homomers form nonfunctional receptors that bind only glycine
(60,
61), and NMDAR2 subunits must coassemble with NMDAR1 for functioning ligand-gated ion channels to result
(62,
63). The expression of certain binding sites is associated with specific NMDAR2 subunits, particularly the NMDAR2A subunit with competitive antagonists of the glutamate site and NMDAR2B with the polyamine site
(63–
65). NMDA receptors containing NMDAR2A or NMDAR2B subunits bind MK-801 more avidly than those containing NMDAR2C or NMDAR2D subunits
(63). In vitro studies have suggested that MDL105,519 labels NMDAR1-containing NMDA receptors
(53), while ifenprodil labels NMDAR2B-containing NMDA receptors
(63,
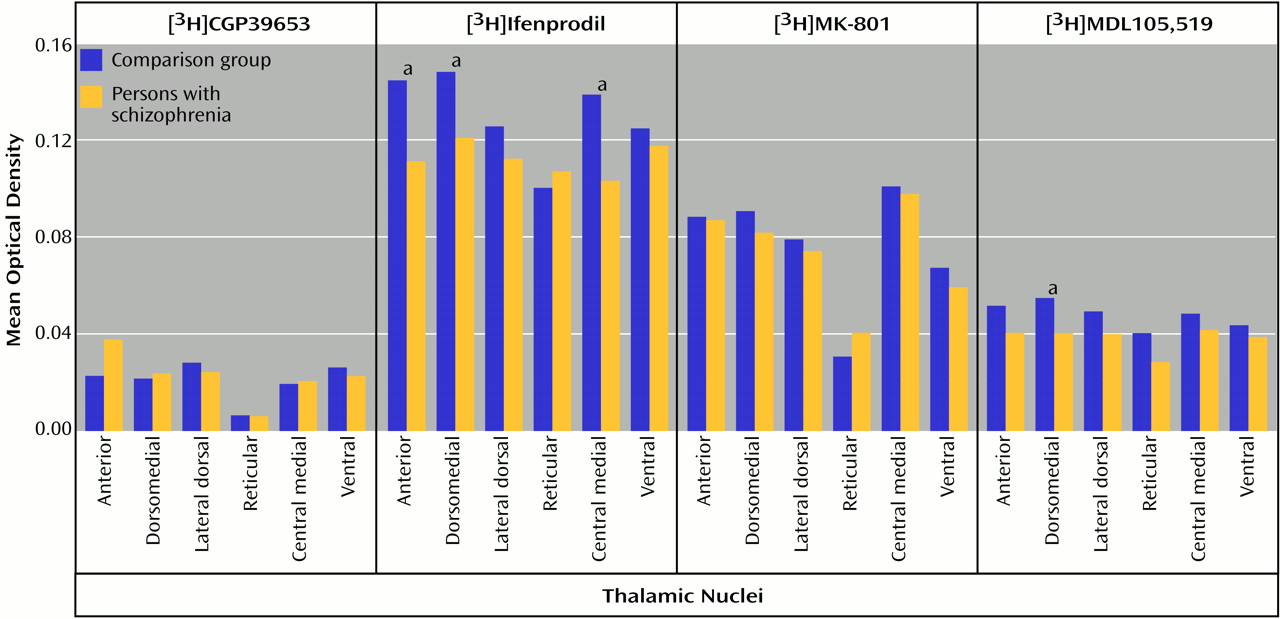
66). In some thalamic nuclei in the current study, there was concordance between the low transcript and binding site levels found in schizophrenia. Both the dorsomedial nucleus (NMDAR1/[
3H]MDL105,519) and the central medial nucleus (NMDAR2B/[
3H]ifenprodil) demonstrate this concordance, which is consistent with a postsynaptic localization of these receptors. These results also show that differences in transcription can vary the pharmacological phenotype of NMDA receptor populations.
Our current data suggest that there is lower glycine binding site expression in some thalamic nuclei in schizophrenia patients than in comparison subjects. Classic pharmacology suggests that high thalamic glycine levels might lead to a down-regulation of this site; there are no data that directly address this possibility. Alternatively, this down-regulation may be a pathological response to normal or low glycine levels, leading to hypoactivity of thalamic NMDA receptors. Perhaps the thalamus is one of the anatomical targets for glycine agonist or partial agonist therapy, which may be compensating for low levels of binding sites by maximizing available glycine sites.
Polyamines also facilitate NMDA receptor-mediated transmission at physiological concentrations. Ifenprodil appears to label a site associated with the polyamine site
(66–
69), so the present data suggest that there is a shift away from polyamine-site-expressing NMDA receptors in the thalamus. As with the glycine site, NMDA receptor neurotransmission may be mitigated in the thalamus in schizophrenia because polyamine facilitation of NMDA receptor currents cannot be fully exploited. Perhaps ligands targeting the polyamine site may also prove therapeutic for schizophrenia.
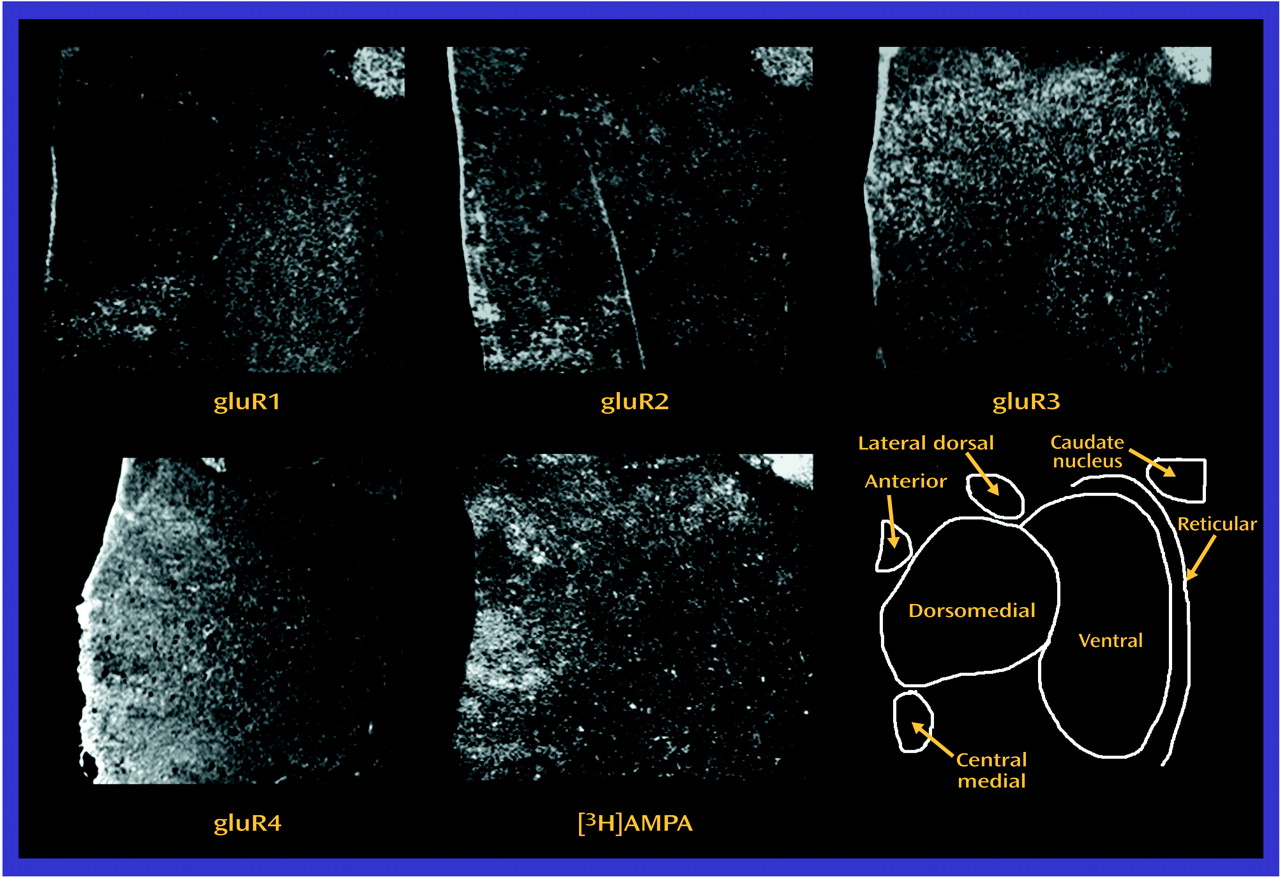
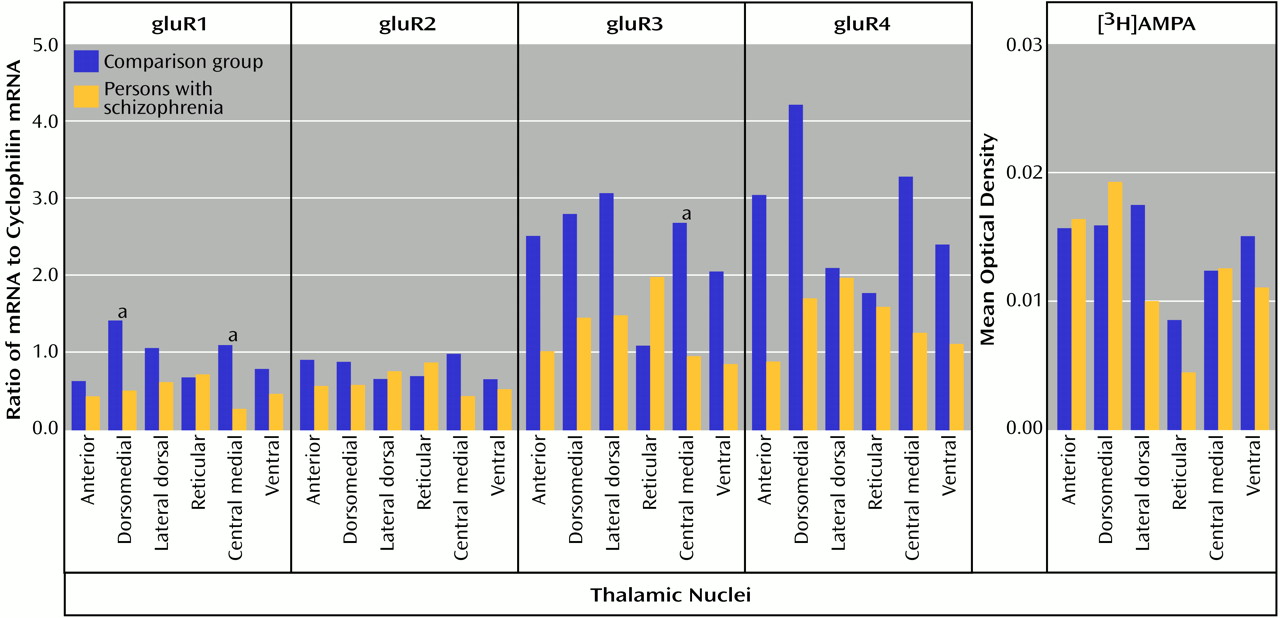
Both AMPA and kainate receptors may facilitate NMDA receptor-mediated neurotransmission; the NMDA hypoactivity postulated in schizophrenia may be associated with differences in AMPA or kainate receptor expression, rather than with a primary problem with NMDA receptor expression. The low levels of gluR1 and gluR3 found, without a difference in [
3H]AMPA binding site levels, suggests that there may be a relatively higher level of gluR2 and gluR4 subunits in assembled AMPA receptors in the thalamus in schizophrenia. Studies have shown that gluR2-containing AMPA receptors have low calcium conductance
(16,
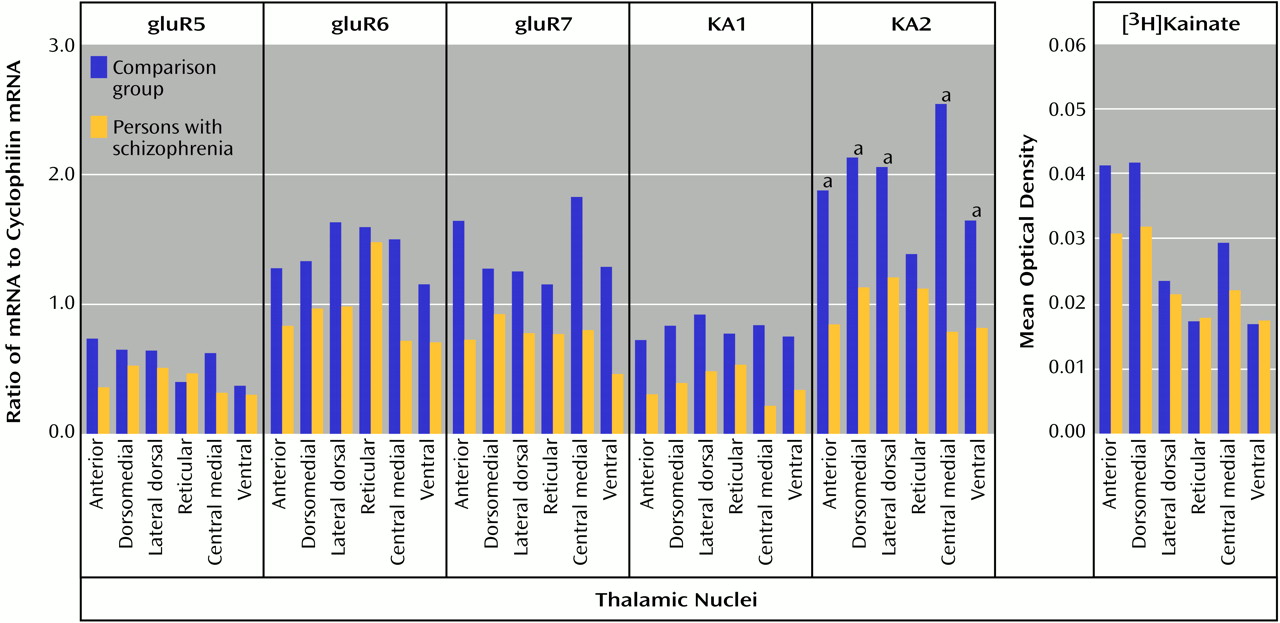
70). Therefore, a lower AMPA receptor-mediated activity in schizophrenia patients than in comparison subjects may lead to a lower facilitation of NMDA receptor activity, which is consistent with our hypothesis. Likewise, the lower amount of KA2 subunits seen in our schizophrenia patients than in the comparison subjects may affect kainate receptor-mediated activity. KA2 subunits do not form homomers, but their coassembly with gluR5 or gluR6 subunits leads to kainate receptors with higher conductances
(71–
73). Consequently, a lower level of KA2 expression may result in a lower facilitation of NMDA receptor activity by kainate receptors. Lower heteroreceptor facilitation of NMDA receptor activity in schizophrenia patients appears to be limited to ionotropic glutamate receptors, since we did not detect any differences in thalamic metabotropic glutamate receptor expression in a separate study of these same subjects
(46). Taken together, these data suggest that subtle differences in AMPA and kainate receptor subunit composition may result in lower heteroreceptor facilitation of NMDA receptor activity in the limbic thalamus in schizophrenia patients than in comparison subjects.
Previous studies have demonstrated a lower neuronal number in some thalamic nuclei in schizophrenia patients than in comparison subjects, which we did not detect using the neuronal marker neuron-specific enolase
(7,
8,
46,
74). We were only able to examine thalamic nuclei at a single cross-sectional level and did not have the entire extent of the nucleus on which to perform stereology or systemic examination of neuron-specific enolase expression. However, the previously demonstrated lower amounts of both neuron number and total thalamic volume in schizophrenia patients than in comparison subjects may not result in overall differences in cell density, which is consistent with our neuron-specific enolase data. Furthermore, we might expect parallel lower levels in all glutamate receptor subunit mRNAs in schizophrenia patients than in comparison subjects if our current results were just a reflection of lower cell numbers. Therefore, we doubt that putative cellular abnormalities in the thalamus in schizophrenia are the explanation for our findings, although our data could be consistent with a selective loss of a subpopulation of thalamic neurons that selectively express NMDAR1 and NMDAR2C subunits.
Lower levels of expression of ionotropic glutamate receptor subunit mRNAs and NMDA receptor binding sites in schizophrenia patients than in comparison subjects appear to be restricted to glutamatergic relay nuclei, which are reciprocally connected with structures that have been implicated in schizophrenia. The centromedial nucleus, which is a major thalamic relay between prefrontal, cingulate, and other limbic cortical areas
(55,
75) and the nucleus accumbens
(76), has significantly lower levels of expression of NMDAR1, NMDAR2B, NMDAR2C, gluR1, gluR3, and KA2 mRNA and [
3H]ifenprodil binding in schizophrenia patients than in comparison subjects. The dorsomedial nucleus, which projects primarily to the prefrontal cortex
(77,
78) and receives input from the amygdala, cortical areas, and the midbrain
(79–
81), shows lower levels of expression of NMDAR1, NMDAR2C, gluR1, and KA2 mRNAs and [
3H]ifenprodil and [
3H]MDL105,519 binding sites in schizophrenia patients than in comparison subjects. The anterior nucleus, which projects to the cingulate gyrus
(82,
83) and receives input primarily from the subiculum
(82,
83), expresses lower levels of NMDAR2C mRNA and [
3H]ifenprodil binding in schizophrenia, and the lateral dorsal nucleus, which receives input from the hippocampus and projects to the cingulate gyrus
(82,
83), shows lower NMDAR2C and KA2 mRNA expression. Our data reveal low levels of KA2 mRNA only in the ventral nuclei of schizophrenia patients, which project to somatosensory, motor, and premotor cortical areas
(55,
84,
85); these regions are probably not implicated in the pathophysiology of schizophrenia. Similarly, our data reveal no lower levels of binding or transcript expression in the reticular nuclei of schizophrenia patients, which utilize GABAergic projections to other thalamic nuclei
(55,
86). There appears to be a continuum of abnormal glutamate receptor expression, with regions projecting to the prefrontal cortex having the greatest degree of abnormality, to nonlimbic thalamic areas having few, if any, abnormalities. These findings suggest that low glutamate receptor expression occurs in the thalamic areas that are components of complex cortical and subcortical circuitry associated with the pathophysiology of schizophrenia.
The hypoactivity of the thalamus seen in imaging studies of schizophrenia may be associated with concomitant low level of neurons. However, our present data suggest that the hypoactivity may also be related to a lower level of NMDA receptor-mediated activity. NMDA receptors are the predominant transducer of glutamatergic neurotransmission in the thalamus
(17), so an illness-associated low level of NMDA receptor activity may manifest as low metabolic activity in the thalamus as well as in its efferent targets.
This study has a number of limitations that should be considered when interpreting these data. As in most studies of schizophrenia that rely on postmortem tissue, there is the potential confounding variable of antipsychotic exposure. Although it is clear that antipsychotic exposure may alter thalamic metabolism and immediate early gene expression
(87–
90), antipsychotics have not been found, at least in one study
(91), to regulate NMDA receptor expression. Nonetheless, some of these results could be due to the effects of chronic antipsychotic treatment. Similarly, these results could be in part attributable to the effects of chronic institutionalization, as this is an older inpatient population; the comparison group was from neighboring nursing homes, and this may at least in part control for some aspects of residential care. It should be appreciated that these data are from an older cohort of subjects, and although the resulting data are perhaps a fair reflection of thalamic neurochemical anatomy in schizophrenia in later life, they may not generalize to younger patients. Accordingly, although these findings are intriguing, whether they are primarily due to schizophrenia or are a secondary condition associated with having this chronic illness for many decades cannot be answered by these results.
Our results demonstrate that glutamate receptor expression in the thalamus of schizophrenia patients is different from that found in the thalamus of comparison subjects at both transcriptional and posttranscriptional levels, but the differences are most prominent in nuclei with reciprocal projections to limbic regions. These results also suggest that differences in NMDA receptor subunit mRNA levels affect the expression of polyamine and glycine binding sites in the thalamus of schizophrenia patients, highlighting the importance of examining receptor expression at multiple levels of gene expression. Combination therapy with positive modulators of both the glycine and polyamine sites of NMDA receptors may prove to be an efficacious treatment strategy for schizophrenia.