In 1937, Papez hypothesized that the expression and decoding of emotion was the function of a circuit that involved the anterior cingulate, hippocampus, fornix, mammillary bodies, anterior thalamus, and hypothalamus. While the anterior thalamus and mammillary bodies are no longer considered to be involved in emotional processes, the amygdaloid complex is now believed to have an emotional function
(1). Whether these brain regions are a part of a circuit or not, a postmortem examination of the anterior cingulate cortex, amygdala, hippocampus, subiculum, entorhinal cortex, mammillary bodies, and septum showed changes in patients with autism
(2). Along with the limbic system, cerebellar architecture is altered in individuals with autism. Alterations in the limbic system, which is implicated in memory, learning, emotion, and motivation, could explain some of the clinical features of autistic disorders.
Despite the evidence from postmortem research that implicates the limbic cortex in autism, neither structural imaging studies nor functional imaging studies have systematically evaluated the structures of the limbic circuit in patients with autism. One recent magnetic resonance imaging (MRI) study reported no difference in the hippocampal volume between autistic individuals and normal volunteers
(3), and another study that examined only the posterior hippocampus had equivocal findings
(4).
Early positron emission tomography (PET) studies have suggested the anterior cingulate gyrus and the basal ganglia as being possible sites for functional alteration in autism
(5–
7), but none had used PET/MRI coregistration for precise anatomical localization of the differences in metabolic activity patterns that distinguished patients from healthy comparison subjects. Muller et al.
(8) recently reported alteration in the thalamic blood flow of autistic patients relative to that of healthy comparison subjects who were performing an expressive language task. As assessed by
15O-water PET, autistic patients also differed from healthy comparison subjects in the blood flow of the dentato-thalamo-cortical pathway, thus confirming the postmortem findings
(2). A more recent functional MRI study reported that relative to healthy comparison subjects, no activation was seen in the amygdala of either patients with Asperger’s disorder or higher functioning autistic patients when trying to decode the emotional expression of another person
(9). Using coregistered PET/MRI, we examined seven patients with autism and seven age- and sex-matched healthy comparison subjects during performance of a serial verbal learning task and reported glucose hypometabolism in the right anterior cingulate cortex in the autistic patients
(10). The autistic patients also had diminished volume of the anterior cingulate, specifically for Brodmann’s area 24′, which has mainly executive functions and is involved in information processing
(11). In the current study, we extended our study group to include patients with Asperger’s disorder and examined both the volume and glucose metabolism of the anterior and posterior cingulate gyri, the amygdala, and the hippocampus. We hypothesized that the amygdala and the hippocampus would show glucose metabolic rate differences in the patients with autism spectrum disorders in accord with the postmortem findings. To the best of our knowledge, this is the first study in which metabolic mapping of limbic circuit structures implicated in autism spectrum disorders has been undertaken.
Method
Subjects
Seventeen patients (15 men, two women; mean age=27.7 years, SD=11.3) with autism (N=10) or Asperger’s disorder (N=7) were recruited for participation in this neuroimaging study from referrals to The Seaver Center for Autism Research at Mount Sinai School of Medicine. The anterior cingulate findings from seven of the autistic patients were published in a preliminary report
(10). The patients had been referred to The Seaver Center by their psychiatrist or neurologist with the possible diagnosis of either autism or Asperger’s disorder. After complete description of the study to the subjects, written informed consent was obtained. Patients were screened to rule out the presence of other neurological disorders, including seizures and head trauma. All patients were verbally fluent, and their full-scale IQ scores ranged from 55 to 125. Thirteen patients had their diagnosis confirmed with the Autism Diagnostic Interview, a semistructured interview for parents of the autistic individual
(12). The remaining four patients were given a diagnosis of autism or Asperger’s disorder following a clinical interview by an experienced child psychiatrist, either because the parents were deceased or were not available for interviewing. All patients met DSM-IV criteria for autism or Asperger’s disorder, both of which are pervasive developmental disorders. However, since no patients with Rett’s or childhood disintegrative disorders were included, the patient group was classified as those with “autism spectrum disorders.” One patient was being treated with nortriptyline at the time of the scan, but the others had not taken psychoactive medications for several months or more.
Healthy comparison volunteers (15 men and two women; mean age=28.8 years, SD=9.4, IQ range=88–136) were recruited from the community around Mount Sinai School of Medicine. They were screened to rule out both the presence of neurological or psychiatric illnesses and a history of psychiatric illnesses in their families. Urinary analyses at the time of the scan revealed the presence of no psychoactive substances.
Imaging
Axial MRI scans were acquired with a 1.5-T GE Signa 5x system (Milwaukee) (three-dimensional spoiled gradient recall acquisition in steady state, TR=24 msec, TE=5 msec, flip angle=40°, contiguous 1.2-mm slices).
As a control of mental activity during the
18F-fluorodeoxyglucose (FDG) uptake period that preceded PET scanning, subjects performed a variant of the California Verbal Learning Test
(13) that was modified for use in this imaging experiment (a 16-word list was presented on a screen, with a 2-second interword interval and four semantic categories)
(14). The test yielded scores for mean number of words correct, perseverations, and intrusions. Subjects were then moved to the PET scanner (full width at half maximum=4.2–4.5 mm), and scans with 3–5 million counts/slice were acquired. Because memory skills are generally intact in patients with autism, the verbal learning test seemed to be an appropriate task to be performed during the FDG uptake period.
Regions of Interest
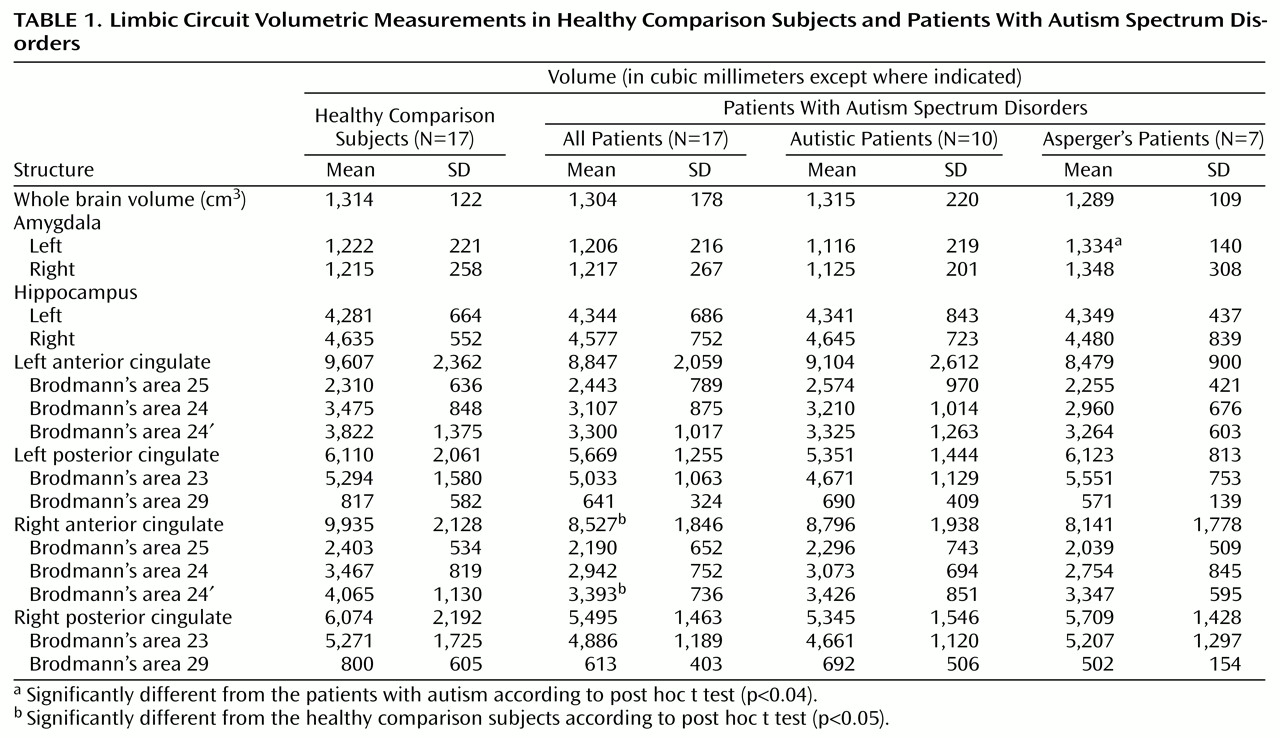
Two researchers, without knowledge of subject diagnosis, outlined the right and left sides of the anterior cingulate gyrus. The area of the anterior cingulate gyrus was measured for seven subjects on three different slices (taken at 25%, 50%, and 75% of the head height); good intertracer reliability was shown (intraclass correlation coefficient [ICC]=0.87). Outlining began ventrally with the plane showing the appearance of the cingulate sulcus in place of the gyrus rectus (at approximately 28% of the head height, z level=–12 to –16 on the Talairach and Tournoux atlas [15]). In the occipital region, posterior cingulate gyrus outlining began with the appearance of the splenium of the corpus callosum. On the axial plane in which the corpus callosum disappears (at approximately 54% of the head height in the Matsui-Hirano atlas [16], or z=32), margins of the anterior and posterior cingulate gyri were defined, and these coordinates were applied to the remaining axial slices dorsally. The anterior cingulate gyrus was operationally defined by outlining that began at the deepest recess of the cingulate sulcus, moved medially, and then moved in an inferior direction until reaching the callosal recess. The two recesses were connected to each other with a straight line, and this triangular area also included the cingulum (
Figure 1).
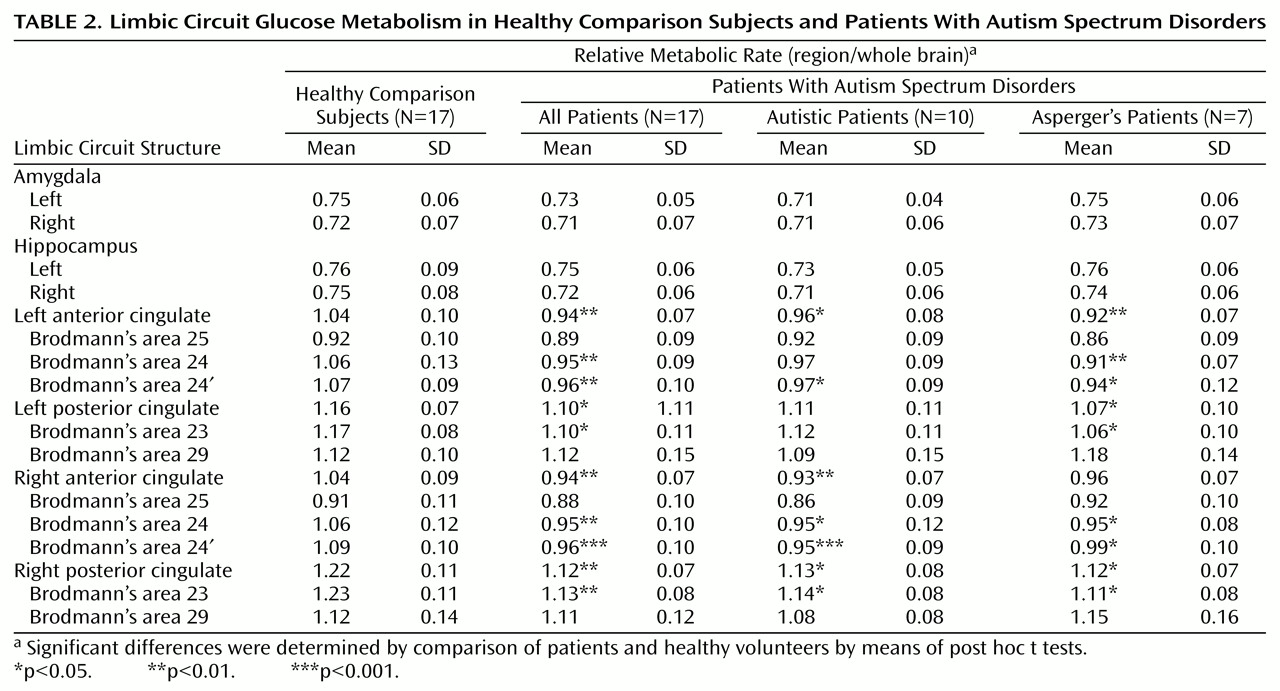
The amygdala was outlined on coronal MRI sections by two researchers, and good intertracer reliability was shown (ICC=0.82, area measured on three slices at the 25th, 50th, and 75th percentiles of anteroposterior distance). Outlining of the amygdala began at its largest extent (approximately the center in the anteroposterior dimension) where clear boundaries between gray matter and surrounding white matter are visible. At this mid-section the amygdaloid complex is roughly elliptical in shape, and anatomical margins are defined by the cornu ammonis and the white matter of gyrus ambiens in the medial aspect, the cornu inferius of the lateral ventricle in the ventral aspect, the temporal lobe white matter laterally, and the gyrus semilunaris in the dorsal aspect. Using an edge contrast-enhancing technique (gradient filter)
(10), we were able to better visualize the dentate gyrus of the hippocampus and boundaries between the hippocampus and the amygdala. The posterior portions of the amygdaloid complex were outlined by using the ventricular recess, hippocampus, and gyrus semilunaris as reference points (
Figure 2).
Outlining the anterior end of the amygdaloid complex posed more difficulty. In the anterior aspect, the amygdaloid complex gray matter is more heterogeneous and hard to identify. We carried out the outlining from the midsection forward by using gradient filtering and completely excluded the entorhinal cortex, which may include the inferior amygdala. The outlining ended at the first coronal MRI section on which there was visible white matter between the amygdala, ambiens, and white matter of the entorhinal cortex. This procedure may have omitted the very anterior end of the amygdaloid complex, but it had the advantage of excluding other extraneous structures from our analysis.
The hippocampus was outlined by one trained researcher who was unaware of the subjects’ diagnoses. Outlining of the hippocampus was done on consecutive 1.2-mm-thick coronal MRI slices. Good intertracer reliability was seen for two tracers who measured the hippocampus in six subjects on three slices at the 25th, 50th, and 75th percentiles of anteroposterior distance (ICC=0.81). The anterior boundary of the structure at the junction with the amygdala was defined by the presence of the temporal horn of the lateral ventricle, lateral and superior to the genu of the hippocampus. Posteriorly, the hippocampus was traced throughout its entire extent, past the level of the pulvinar, until it was no longer visible. In cross-section, the hippocampus was traced to include all regions of Ammon’s horn as well as the dentate gyrus (
Figure 2). For each structure, both an absolute volume in cubic centimeters and a relative value (structure volume/whole brain volume) were calculated.
Statistical Methods
After PET/MRI coregistration, region of interest coordinates were applied to the PET scan of each individual, and metabolic three-dimensional significance probability maps
(17) of t tests comparing the groups for the four structures were reconstructed. All regions of interest were adjusted to the mean normal number of slices by interpolation. Each individual’s region of interest was then warped to an averaged contour of the normal group by adjusting the length of the line joining the centroid and the contour edge at 720 angular positions to the group length average. Between-group differences in the metabolism of the anterior cingulate gyrus, posterior cingulate gyrus, amygdala, and hippocampus were assessed by repeated measures analysis of variance (ANOVA) and follow-up t tests on volume and mean relative metabolic rate. This design allowed testing of the hypothesis of a deficit in the entire limbic circuit (main effect of group) with a single F test or a deficit in only a portion (group-by-region interaction). We present post hoc t test contrasts for comparison with published regional postmortem data. We also performed three-dimensional significance probability mapping with resampling to control against type I errors. The resampling strategy adapted from cluster-counting approaches
(18,
19) used actual PET scan data (instead of Monte Carlo simulations) from normal individuals performing the same verbal learning task. To establish the threshold for significance in a t test analysis of 17 healthy comparison subjects and 17 patients with autism spectrum disorders, two groups (N=17 in each) were drawn randomly from our pool of 34 normal subjects, a procedure adopted to match our earlier report
(10). Note that standard deviations in normal subjects are larger than or similar to those in patients, making this approach relatively conservative for type I errors. For each randomly chosen group, pixel-by-pixel t tests were performed. The image was thresholded at the p<0.05 level (t=2.04, df=32, p<0.05). In each cluster of pixels above the threshold, the number of contiguous pixels was counted, and the volume of the largest cluster (the number of contiguous pixels multiplied by the average difference between the t value for each pixel and 2.04, which was the t value at which p<0.05) was determined (e.g., 100 pixels with a mean t value of 2.68 yield a volume of 64). An empirical table of cluster volumes was created by generating 5,000 such random samples from the 17 patients with autism spectrum disorders and the 17 healthy comparison subjects and obtaining the single largest cluster for each random draw. Volumes for 95%, 97.5%, and 99% levels were obtained, which permitted a test of whether any given pixel cluster volume might have occurred by chance if the subject groups differed no more than randomly chosen sets of healthy comparison subjects.
Results
MRI Volume Analysis
Although previously reported by other researchers
(20,
21), group differences in whole brain volume were not found in the current study. Neither the absolute nor the relative volumes of the amygdala and hippocampus differed between patients with autism spectrum disorders and the healthy comparison subjects. Greater left amygdalar volume was seen in the patients with Asperger’s disorder than in the autism patients (
Table 1).
The anterior cingulate gyrus consists of three functionally distinct regions. Brodmann’s area 25 has emotional processing functions and participates with Brodmann’s area 24 in the modulation of motor responses to emotional cues. Brodmann’s area 24′ is involved in information processing and execution of higher cognitive functions
(11). We divided the anterior cingulate into three segments (Brodmann’s areas 25, 24, and 24′) and divided the posterior cingulate into two segments (Brodmann’s areas 23 and 29) on the basis of proportions derived from the Talairach-Tournoux atlas
(15). An analysis of covariance that involved all the limbic circuit structures with brain volume as a covariate was nonsignificant. Both absolute and relative volumes of the right anterior cingulate gyrus were smaller, as assessed by t tests, in the patients with autism spectrum disorders than in the healthy comparison subjects (
Table 1). Furthermore, in the right anterior cingulate, Brodmann’s area 24′ was significantly smaller in the patients with autism spectrum disorders than in the healthy comparison subjects. There were no group differences in left anterior cingulate gyrus volume nor were there posterior cingulate gyrus volume differences between the two autism spectrum disorder groups (
Table 1).
PET Glucose Metabolism Analysis
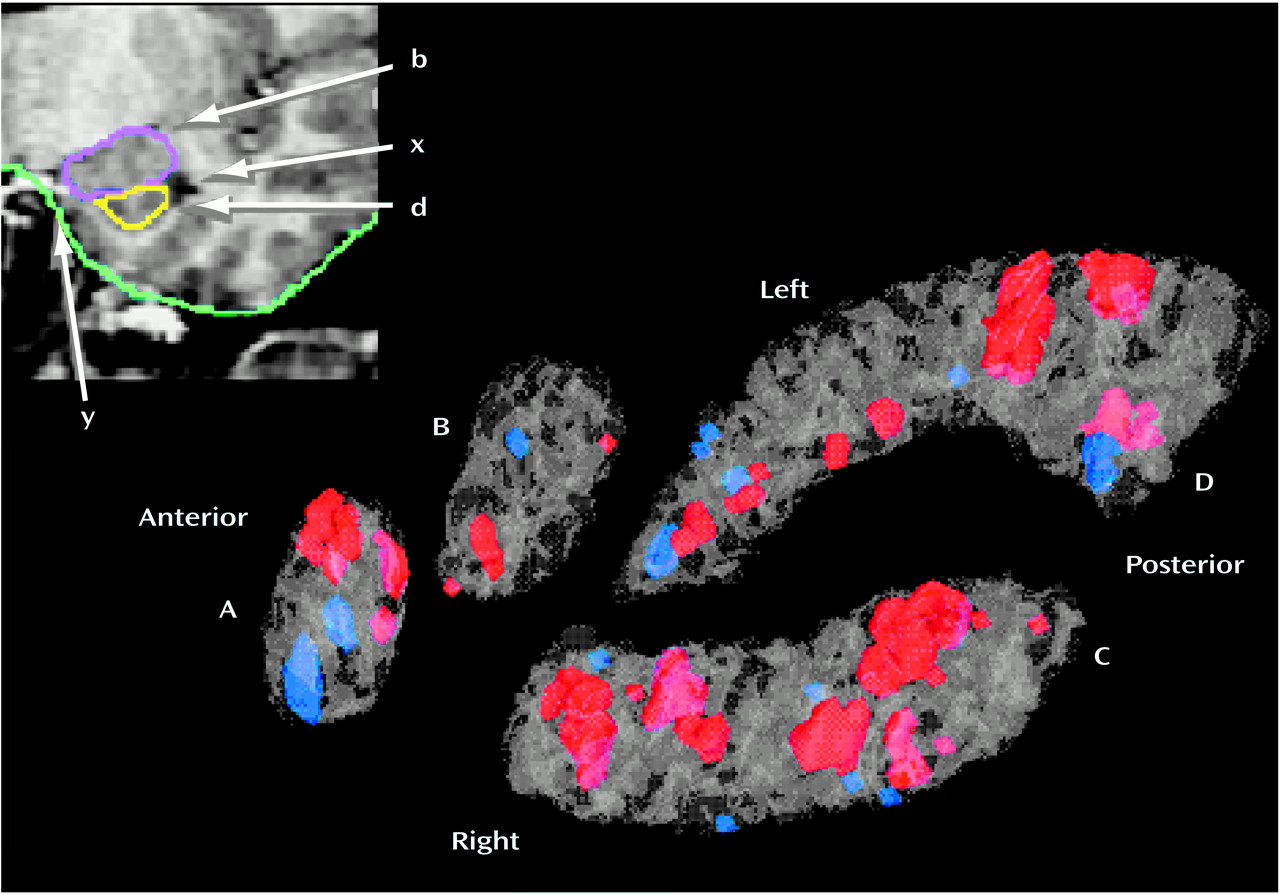
We did not find significant group differences in the glucose metabolic rate of the amygdala or the hippocampus as assessed by significance probability mapping (
Figure 2) or by t tests on these regions of interest (
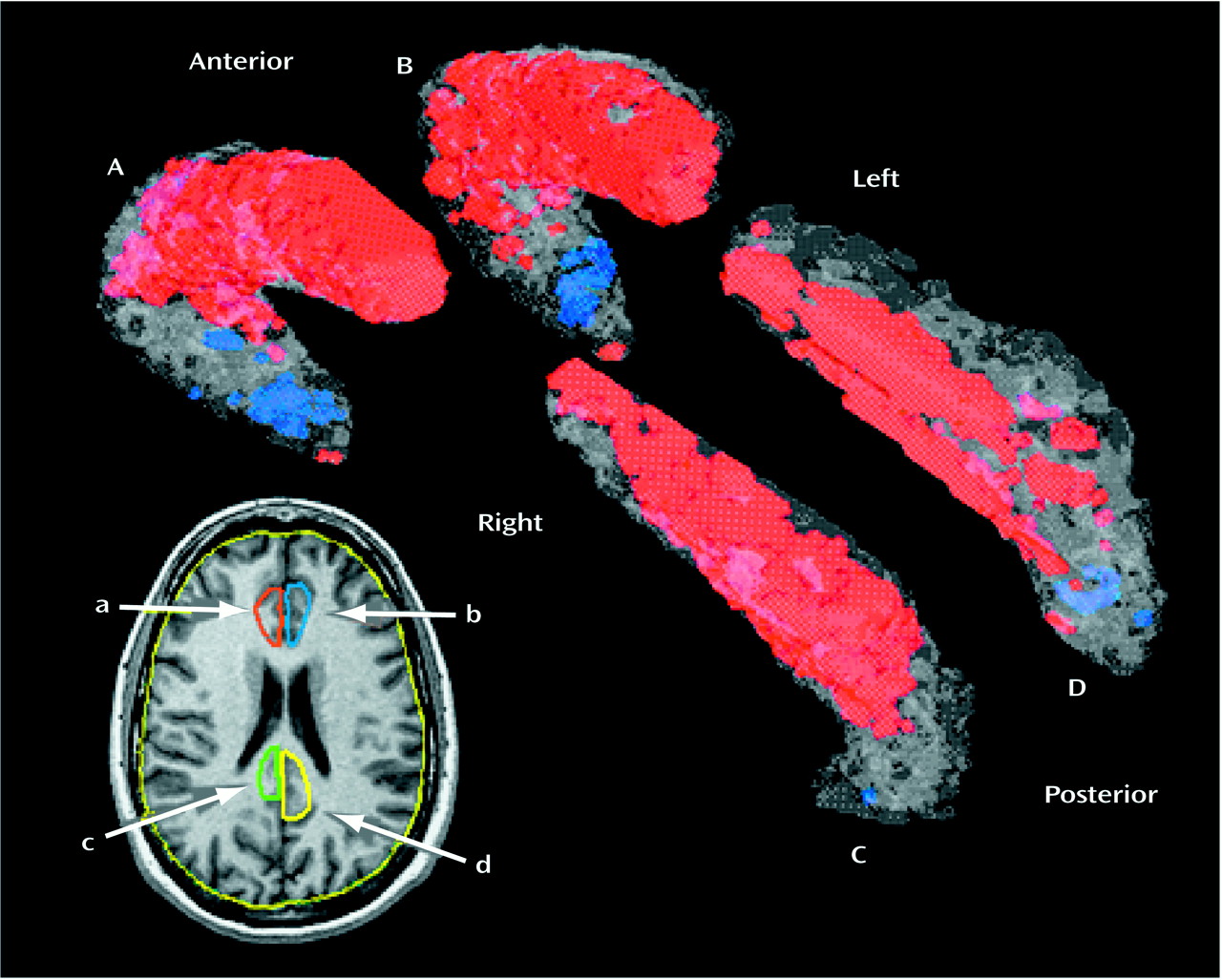
Table 2). Furthermore, dividing the autism spectrum disorders group into the autistic and Asperger’s subgroups also failed to reveal significant between-group differences in these regions. By contrast, significance probability mapping (
Figure 1) showed that glucose metabolism in the right (cluster volume=3,144; maximum t in cluster=5.03) and the left (volume=1,556; maximum t=5.68) anterior cingulate gyrus was significantly lower in the patients with autism spectrum disorders than in the healthy comparison subjects (t=2.03, df=33, p<0.01). The Asperger’s patients exhibited bilateral glucose hypometabolism in the anterior cingulate cortex (left volume=2,288; maximum t=6.46 [t=2.07, df=23, p<0.01]; right volume=2,063; maximum t=7.18 [t=2.07, df=23, p<0.02]) relative to the healthy comparison subjects, whereas autistic patients differed only in the right anterior cingulate gyrus (volume=2,864; maximum t=6.17 [t=2.06, df=26, p<0.01]). The patients with autism spectrum disorders also showed lower glucose metabolism in the right and left posterior cingulate gyrus (left: volume=535; maximum t=5.71 [t=2.03, df=33, p<0.02]; right: volume=978; maximum t=5.42 [t=2.03, df=33, p<0.01]). Within the two diagnostic subgroups, hypometabolism was limited to the right posterior cingulate gyrus in autistic patients (volume=461; maximum t=4.86 [t=2.06, df=25, p<0.05]), whereas hypometabolism was again bilateral in the Asperger’s patients (left: volume=1,395; maximum t=7.82 [t=2.07, df=22, p<0.01]; right: volume=1,922; maximum t=7.56 [t=2.07, df=22, p<0.01]).
In addition to significance probability mapping, we also assessed potential group differences by performing an ANOVA for the group-by-hemisphere-by-region of interest (Brodmann’s areas 25, 24, 24′, 23, and 29 as well as the hippocampus and amygdala) interaction. Only the group-by-region of interest interaction was significant (F=4.46, Huynh-Feldt adjusted df=4.34, 138.92, p<0.002). The same ANOVA carried out with only the 10 new patients that were added to our original group of seven autistic patients
(10) similarly yielded a significant group-by-region of interest interaction (F=5.40, df=4.36, 109.06, p=0.0004). To control for differences in task performance between the healthy comparison subjects and the patients with autism spectrum disorders, we performed the same analyses for a subgroup of nine autism spectrum disorder patients (three with autism and six with Asperger’s disorder) who performed at the level of the 17 comparison subjects (13.7 and 13.9 words correct, respectively). Subjects were matched by their task performance, and similar results were obtained (group by region of interest: F=5.03, df=4.21, 101.02, p=0.0008). Post hoc t tests (N=17) showed bilateral glucose hypometabolism in patients with autism spectrum disorders relative to the comparison subjects in Brodmann’s areas 24, 24′, and 23 but not in Brodmann’s areas 25 or 29 or in the hippocampus or amygdala (
Table 2). In contrast with the results of the three-dimensional significance probability maps, the post hoc region-of-interest t tests did not show significant differences between the Asperger’s and autism patients.
Task Performance and Metabolic Correlates
One autistic patient was not included in this analysis because he failed to perform the task (i.e., the subject repeated the words out loud but did not recall more than one word in the list). Healthy comparison subjects correctly remembered a higher number of words during the verbal learning trials (mean=13.9, SD=1.3) than did the patients with autism spectrum disorders (mean=11.0, SD=3.9) (t=2.78, df=18.2, p<0.02; Levene F for variability=10.8, df=1, 31, p<0.003). The two groups did not differ in perseverative errors or intrusion scores, but the healthy comparison subjects were better in semantic categorization of the word lists (mean=8.8, SD=2.6) than were the patients with autism spectrum disorders (mean=5.3, SD=3.7) (t=3.16, df=31, p<0.004; Levene F=3.69, df=1, 31, p<0.07).
On an exploratory basis, we examined correlations between metabolism in the key cingulate areas and task performance. For the healthy comparison subjects, glucose metabolic rates in right Brodmann’s area 25 (r=0.60, df=15, p<0.05), right Brodmann’s area 24 (r=0.51, df=15, p<0.05), and the entire right anterior cingulate gyrus (r=0.49, df=15, p<0.05) correlated with the number of words remembered correctly. For the patients with autism spectrum disorders, correlations between glucose metabolism and the number of correct words were not specific to Brodmann’s areas but were found for the entire right anterior cingulate gyrus (r=0.54, df=14, p<0.05). No correlation reached the p<0.01 level, survived Bonferroni correction, or showed p<0.05 differences between the patients with autism spectrum disorders and the healthy comparison subjects.
Clinical Correlations
Correlations between limbic circuit components and major clinical symptom severity assessments from the Autism Diagnostic Interview were calculated on an exploratory basis. Thirteen of the patients with autism spectrum disorders were evaluated with the Autism Diagnostic Interview for diagnostic purposes (but one Autism Diagnostic Interview was not available for scoring). Autism Diagnostic Interview subscores (social interaction, verbal communication, nonverbal communication, restricted interest domains, point in childhood at which the patient was worst) were entered into a correlation analysis with glucose metabolic rates. Glucose metabolism in left Brodmann’s area 24 correlated with social interaction (r=0.83, df=10, p<0.01), verbal communication (r=0.64, df=10, p<0.05), and nonverbal communication (r=0.68, df=10, p<0.05) subscores. Metabolism in left Brodmann’s area 24′ (r=0.65, df=10, p<0.05) and left Brodmann’s area 23 (r=0.64, df=10, p<0.05) correlated with social interaction subscores. Metabolism in the entire left anterior cingulate gyrus correlated with social interaction (r=0.82, df=10, p<0.01), verbal communication (r=0.68, df=10, p<0.05), and nonverbal communication domains of the Autism Diagnostic Interview (r=0.64, df=10, p<0.05). It is of interest that the only inverse correlation was between the relative volume of the left amygdala and the Autism Diagnostic Interview nonverbal communication domain (r=–0.62, df=10, p<0.05).
Discussion
A recent MRI study by Aylward et al.
(22) reported diminished volumes of the amygdala and hippocampus in a group of high-functioning autistic patients. Although we hypothesized both volumetric and metabolic changes in the amygdala and hippocampus in our group of high-functioning patients with autism spectrum disorders, we did not find any significant differences for these structures compared with findings in healthy volunteers. The absence of significant group differences in hippocampal volume is in accord with some earlier studies
(3,
4). Our findings may also reflect the characteristics of our patient population: none had seizure disorder, which is known to affect hippocampal size
(23). Moreover, all patients had verbal communication skills and were relatively high functioning; as such, our study group is representative of 25% of the patients with autism. The lack of group differences in glucose metabolic rates in the amygdala and hippocampus in our study may also be task-related. Although the hippocampus is involved in memory function, serial verbal learning is believed to reflect primarily working memory and may not require hippocampal activity
(13). The use of the verbal memory task as an uptake condition, however, has the advantage of minimizing individual differences in episodic memory retrieval that may occur during an unstructured “resting” period
(24).
The main findings in this study were the reduced glucose metabolism in the entire cingulate cortex in patients with autism spectrum disorders and the reduced volume of the right anterior cingulate gyrus, specifically Brodmann’s area 24′. When we attempted to localize metabolic findings by dividing the cingulate into its functional subregions, we found bilateral glucose hypometabolism in Brodmann’s areas 24, 24′, and 23 in the patients with autism spectrum disorders. Brodmann’s area 24 is involved in motor responses to emotional cues and would not be expected to be involved in verbal memory. On the other hand, because Brodmann’s area 24′ is implicated in information processing and higher cognitive functions, semantic categorization and incongruent word suppression may have common efferents and may be related to this area’s function
(25,
26). Little is known about Brodmann’s area 23, but it also has memory functions, acts as a relay area among the associative cortices, and is involved in spatial orientation
(27). The lack of alterations in metabolism for Brodmann’s area 25 is interesting, since it is known to have emotional functions, gives emotional color to the higher executive functions, and is affected in depression
(28,
29). Because of a generally dull affective coloring in autistic patients, one might have expected functional changes in the subgenual cingulate cortex in the patients, which did not prove to be the case in this cohort.
The subgroup of patients with Asperger’s disorder emerged as physiologically distinct. When the seven subjects with Asperger’s syndrome were removed from the cohort, the remaining autistic patients continued to be characterized by hypometabolism voxel clusters in the right anterior and the right posterior cingulate gyrus as determined by the three-dimensional resampling method. Note, however, that ANOVA interactions containing both group and hemisphere did not reach significance and that our three-dimensional region-of-interest method provided no statistical contrast of laterality. Furthermore, results of post hoc t tests were not consistent with the resampling findings. As determined by the three-dimensional resampling method, Asperger’s patients showed bilateral cingulate hypometabolism relative to healthy comparison subjects. Another interesting finding was the amygdala volumetric difference between the Asperger’s and the autistic patients. The Asperger’s patients had larger left amygdala volume, and the volume of the amygdala in all patients, but not normal volunteers, was correlated with semantic categorization scores and total number of words correct. In addition, larger left amygdala volumes were associated with lower Autism Diagnostic Interview nonverbal communication scores. Taken together, these exploratory correlations suggest that volume reduction in the left amygdala may be related to poor outcome in patients with autism. It raises the possibility that larger amygdala size in Asperger’s patients might compensate for diminished cingulate function or that the larger amygdala might suppress cingulate function. Thus, the metabolic data for the anterior cingulate are consistent with an autism spectrum (autism-Asperger-normal), and the volumetric data for the amygdala are consistent with separate entities within the category of pervasive developmental disorders.
Our findings of lower cingulate metabolism in autistic patients do not appear to be secondary to their poorer verbal memory performance. An analysis of normal subjects and a performance-matched autistic subgroup yielded the same significant decreases in cingulate metabolism. Compensatory verbal memory processing in posterior cingulate (Brodmann’s area 29), where modest but nonsignificant elevations were seen in autistic patients, or in other areas such as the cerebellum or cerebral cortex
(2,
8,
30) not assessed in this study, should remain a goal in future studies.