Panic disorder has been associated with numerous claims of brain dysfunction, but the neurobiology of the development of acute panic attacks remains largely unclear. Interest has focused on the potential role of the hypothalamic-pituitary-adrenal (HPA) axis in the pathophysiology of panic disorder; however, findings have been inconsistent
(1). Increased cortisol levels have been found in normal individuals under stress provocation
(2) and have been associated with altered HPA function due to stress
(3). In patients with panic disorder, however, investigators have failed to find any increase in cortisol during panic attacks induced by lactate infusion
(4–
6) or carbon dioxide inhalation
(5,
7). On the other hand, in patients who experience panic after lactate infusion, a rise in cortisol was found before the infusion
(8).
In the study reported here, saliva collection was used to provide samples for measuring concentrations of cortisol noninvasively during unprovoked panic attacks in the patients’ natural environment.
METHOD
The study design was approved by the institutional ethics committee. Subjects were consecutively admitted outpatients with panic disorder with or without agoraphobia according to DSM-IV.
Severity of illness was measured by using the Panic and Agoraphobia Scale
(9). Patients were eligible for the study if they had a severity score of at least 18 on the clinician-rated version of the scale. Exclusion criteria were other severe psychiatric or medical illnesses, pregnancy, and intake of contraceptive pills. Patients had to discontinue treatment with any psychotropic drugs at least 2 weeks before the study; there was no substantial benzodiazepine withdrawal in any patient.
Mean severity scores for the patient group were compared with those for a normative sample of 235 patients with panic disorder described elsewhere
(9). After the study had been explained to the subjects, their written informed consent was obtained.
Subjects were given a set of 14 Salivette sampling devices (Sarstedt Inc., Rommelsdorf, Germany) to take with them for use in the event of an acute panic attack. They were instructed to take a saliva sample at the beginning of the panic attack and at 5, 10, 15, 20, 25, 30, 45, 60, 120, and 180 minutes after the beginning of the attack. To obtain comparison values, patients were instructed to take three samples separated by 15-minute intervals, starting 24 hours after the attack occurred. If a panic attack occurred at this point, the patients were to collect the comparison saliva samples another 24 hours later. Patients were instructed to put the collected samples into the freezer compartment of their refrigerator. Patients recorded acute panic symptoms by using the Acute Panic Inventory
(10), a questionnaire with 17 questions that are answered on a 0–3 Likert scale (0=none to 3=severe). Only attacks with an Acute Panic Inventory score of 10 or higher were evaluated. Patients were to record whether they had drunk coffee or alcohol, had smoked, or had bleeding gums in the last hour before taking the samples, as these conditions could lead to changes in cortisol levels. Samples collected under these conditions were excluded from evaluation.
Of 51 patients who were given saliva tubes, 35 provided saliva samples associated with a panic attack. Although patients were given careful instructions, some subjects delivered incomplete sets of saliva samples. The samples of 10 of the 35 patients were excluded from the analysis for the following reasons: 1) insufficient amounts of saliva in samples (five patients), 2) missing comparison samples (three patients), and 3) an Acute Panic Inventory score of less than 10 (two patients). We evaluated one attack per patient, so a total of 25 panic attacks among 25 patients were examined. Sixteen patients were female. Twenty had panic disorder with agoraphobia, and five had panic disorder without agoraphobia. The mean age was 32.1 years (SD=8.1). The mean total scores on the Panic and Agoraphobia Scale, clinician-rated and self-rated versions, were 27.1 (SD=6.0) and 26.9 (SD=5.5), respectively. Eight patients had discontinued their psychotropic medication to participate in the study.
Saliva samples were stored at –80°C, thawed before analysis, centrifuged, extracted by using 1,350 µl ethanol, and analyzed with radioimmunoassay (Scintillation Proximity Assay, Amersham-Buchler, Braunschweig, Germany). The sensitivity of the assay was 37.5 pg/ml. The interassay variance was less than 10%. Recovery values of the extraction procedure were 85% on average.
Data were analyzed with the Statistical Analysis System (SAS 6.12, SAS Institute, Heidelberg, Germany). The areas under the curve for cortisol level versus time for 30 and 60 minutes after the beginning of the panic attack and for the comparison values were calculated by using the trapezoidal method and compared with a modification of the Wilcoxon-Mann-Whitney test for the generalized Behrens-Fisher problem
(11).
RESULTS
According to patients’ records, the panic attacks analyzed in this study lasted 30.7 minutes on average (SD=13.5, range=12–60). The Acute Panic Inventory scores for these attacks averaged 22.3 (SD=8.3, range=10–40).
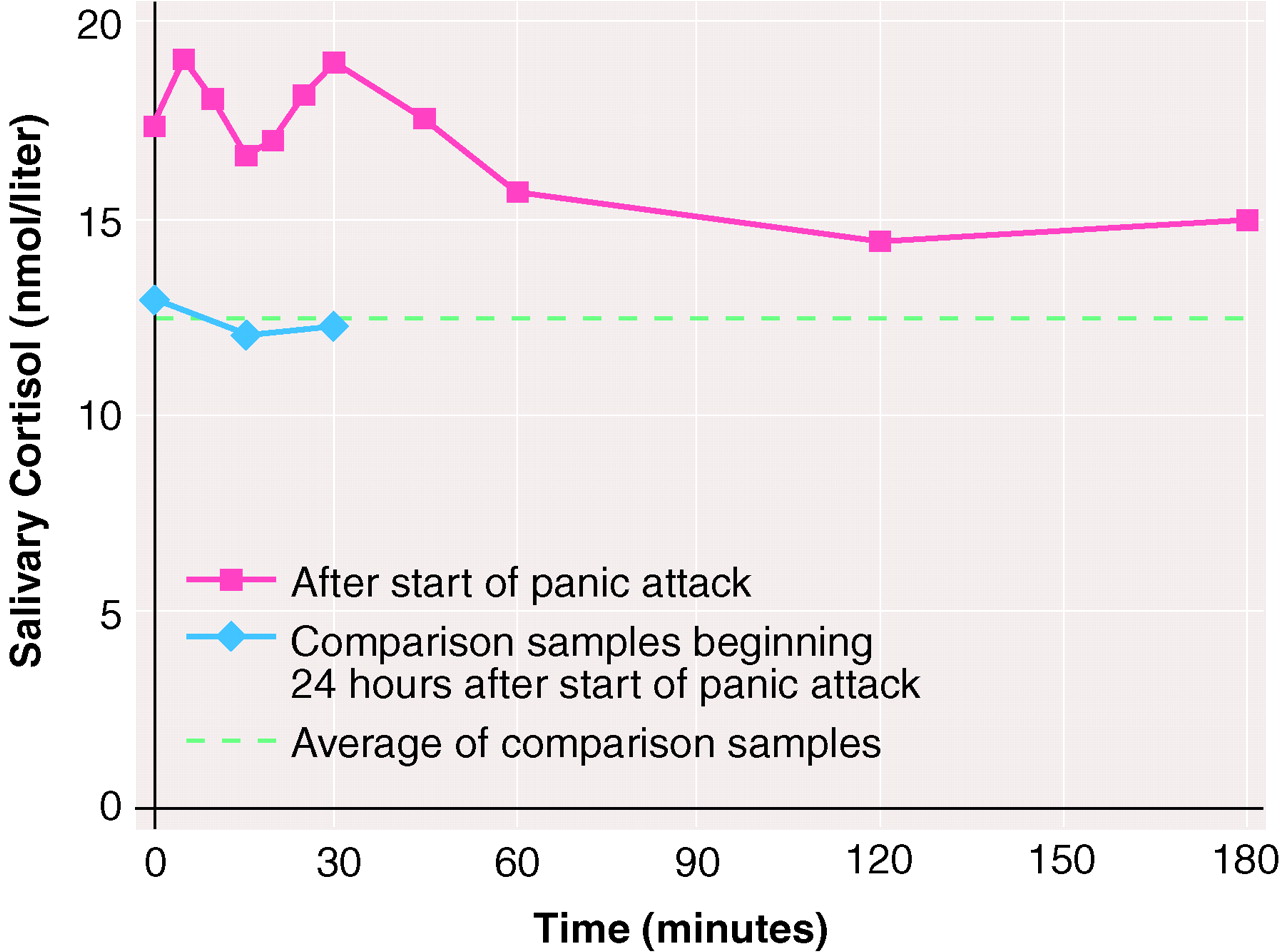
Mean salivary cortisol levels during the period after the start of a panic attack and during the comparison period 24 hours later are shown in
figure 1.
The areas under the curve for cortisol level versus time for the first 30 minutes after the beginning of the panic attack were significantly larger than the areas under the curve for the three values obtained during the 30-minute comparison period (area under the curve of mean cortisol levels for the first 30 minutes divided by 30=17.8, SD=7.92; area under the curve for the comparison period, divided by 30=12.3, SD=5.43; modified Wilcoxon-Mann-Whitney test, B=4.44, N=25, p<0.0002). Also, the mean cortisol values for the first hour after the attack were higher than the comparison values (area under the curve of mean cortisol levels during the first 60 minutes divided by 60=17.6, SD=7.5; B=3.98, N=25, p<0.0005). The mean cortisol values obtained at 2 hours (14.40 nmol/liter, SD=9.90) and 3 hours (14.95 nmol/liter, SD=9.73) after the beginning of the panic attack did not differ significantly from the averaged comparison values (12.36 nmol/liter, SD=5.49) (t=0.87, df=24, p=0.4, n.s., and t=1.1, df=24, p=0.28, n.s., respectively).
No significant correlations were found between the relative cortisol elevation during the panic attack (the ratio of the area under the curve for the attack and the area under the curve for the comparison period) and the severity of the attack as measured by using the Acute Panic Inventory (Spearman rank correlation rs=0.06, N=25, p=0.77, n.s.) or the severity of illness as measured by using the Panic and Agoraphobia Scale (rs=0.18, N=25, p=0.38, n.s.).
The severity of illness in the 25 patients in the study group was higher than in a normative sample of 235 unselected patients with panic disorder (mean Panic and Agoraphobia Scale score=27.5, SD=7.3, compared with mean=23.7, SD=10.8) (Wilcoxon-Mann-Whitney test, U=3,010, p<0.01).
DISCUSSION
In contrast to most other investigations of cortisol changes in panic attacks, this study found elevated salivary cortisol levels during spontaneous panic attacks in a naturalistic setting. Use of the naturalistic setting was important because chemically induced panic attacks may differ from genuine panic attacks.
In two previous studies investigating cortisol in nonchemically induced panic attacks, cortisol elevations during panic attacks were not significant. In one study
(12), panic disorder patients were exposed to phobic situations while blood was sampled from an intravenous cannula. In the other
(13), cortisol was measured during spontaneously occurring panic attacks while patients were confined to bed and blood was sampled by using an indwelling venous catheter. In the first study, patients had to be accompanied to the exposure site by the research psychiatrist; in the second, patients had to stay in the laboratory. In both studies, the possibility that subjects’ anxiety was attenuated by the presence of medical personnel cannot be ruled out, because a main feature of panic disorder is patients’ assumption that they have an organic disease that requires immediate medical support. Furthermore, the previous studies measured the total fraction of plasma cortisol (both free and bound by corticosteroid-binding globulin), whereas our study measured salivary cortisol, which highly correlates with free plasma cortisol
(14), the biologically active fraction
(15). Neither decreased nor increased flow rate has been shown to have any influence on salivary cortisol concentrations
(16).
Although the saliva sampling technique we used may be a noninvasive, stress-free, and easy-to-perform method for studying endocrine abnormalities in panic disorder, some methodological problems must be discussed. Taking saliva samples every five minutes might have distracted the patients, resulting in attenuated panic anxiety. Moreover, measurements may have been influenced if patients preferred to obtain saliva samples for panic attacks that occurred at home and not in typical agoraphobic situations to avoid embarrassment associated with using the sample swab while among other people.
Other methodological problems of this study included the low return rate for the sample sets and the lack of control on subjects’ compliance with the sampling instructions, on their food intake before collecting saliva samples, or on their emotional states when the comparison saliva samples were collected.
We found that subjects’ cortisol levels were already elevated at the first measurement point. It is not clear whether this elevation reflects an anticipatory rise in cortisol that is present before the patient realizes a panic attack is occurring or is due to the time that elapsed between the patient’s becoming aware of the attack and preparing for saliva sampling.
The reasons for the cortisol elevation remain unclear, as only limited data are available on the neurobiological changes that occur during unprovoked, spontaneous panic attacks. The cortisol rise might reflect the increased stress experienced during an attack. Alternatively, it might be an expression of a dysregulation in HPA axis function involved in the pathogenesis of panic attacks, but this explanation is less likely. Additional studies using the naturalistic setting approach are needed.