Dopamine neurotransmission abnormalities have been implicated in the etiology of bipolar disorder
(1,
2). Stimulants such as amphetamine that increase dopamine and norepinephrine release have behavioral effects that resemble mania
(3). Dopamine receptor antagonists are effective antimanic agents
(1,
2). However, whether the dopamine abnormality in bipolar disorder is due to increased presynaptic dopamine production and release or increased postsynaptic receptor response remains unclear. This distinction is important because it can help focus research efforts toward the most likely site of dopamine neurotransmission abnormality in bipolar disorder.
Neuroimaging techniques such as single photon emission computed tomography (SPECT) and positron emission tomography (PET) have made it possible to directly investigate, in vivo, neurochemical synaptic events. Amphetamine challenge has been shown to reduce the binding potential (the product of the density and affinity of available receptors not occupied by dopamine) of the dopamine (D
2/D
3) receptor tracer iodobenzamide ([
123I]IBZM)
(4–
6). Since amphetamine itself does not bind to dopamine receptors, it has been postulated that this effect is mediated by increased dopamine release and displacement of [
123I]IBZM-specific binding
(4,
5).
In humans, it has been shown that after 3 hours of simultaneous administration of a bolus dose and start of constant infusion, a prolonged state of equilibrium of [
123I]IBZM binding to striatal receptors is established
(5). Occipital binding also remains stable. Under these conditions, striatal activity provides a stable baseline against which effects of pharmacological agents, such as amphetamine, can be assessed
(5). With this bolus and constant infusion experimental paradigm, patients with schizophrenia have been shown to have greater amphetamine-induced displacement of [
123I]IBZM binding than was seen in healthy subjects, which suggests a greater dopamine release in schizophrenia
(7,
8).
The dopamine system has been extensively studied using PET and SPECT in schizophrenia, Parkinson’s disease, and, to a lesser extent, depression. However, SPECT and PET neuroreceptor studies in bipolar disorder are scarce. In one of the only studies of its kind, Pearlson and colleagues
(9) used the D
2 receptor radiotracer [
11C]n-methylspiperone and reported that D
2 receptor binding was increased in schizophrenic and psychotic manic patients but not in nonpsychotic manic patients
(9).
One reason for the paucity of neuroreceptor studies in bipolar disorder is that it is difficult to do these studies with acutely ill manic or depressed patients. Drug-free and substance-abuse-free patients are difficult to recruit; informed consent is difficult to obtain; and patients have difficulty complying with the scanning procedures. Furthermore, any abnormality is difficult to attribute solely to the acute mood state versus the underlying pathological state associated with bipolar disorder. Studying euthymic bipolar patients can circumvent some of these problems. Euthymic bipolar disorder patients are usually high functioning, able to give informed consent, and able to comply with experimental protocols. Furthermore, state-related confounds are avoided, and abnormalities uncovered in the euthymic state are more likely to reflect the underlying diathesis in bipolar disorder pathophysiology. In this study, we investigated the differences in amphetamine-induced dopamine release in euthymic bipolar disorder patients and healthy comparison subjects.
Method
Subjects
Approval for the study was obtained from the Yale School of Medicine institutional review board. Subjects and patients were recruited by advertisement and were paid to participate in the study. Eligibility for the study was determined after a detailed interview (to determine history of psychiatric illness and use of psychotropic medications, including neuroleptics), physical examination, blood tests (including a pregnancy test), and an ECG. A urine toxicology screen was also done before the study to rule out recent substance use. This toxicology screen tested for the most commonly used substances, including cocaine, marijuana, amphetamines, benzodiazepines, and opiates.
The patients with bipolar disorder had to meet the following criteria for inclusion in this study: 1) 21–45 years of age; 2) a diagnosis of bipolar disorder determined by the Structured Clinical Interview for DSM-IV; 3) euthymic for more than 4 weeks; 4) no history of substance abuse or dependence in the preceding year; 5) no antidepressant medications in the preceding 3 months; 6) no treatment with neuroleptics in the preceding 6 months; 7) no significant medical illness; 8) no current suicidal or homicidal ideation; 9) able to give informed consent; and 10) not pregnant or lactating. The healthy comparison subjects had to meet the following criteria: 1) no current or past psychiatric or neurological illness, 2) no significant medical illness, 3) not pregnant or lactating, and 4) able to give informed consent. The healthy comparison subjects were matched to the bipolar disorder patients as a group by age and gender. After complete description of the study to the subjects, written informed consent was obtained.
Scan Protocol
SPECT experiment
Bipolar disorder patients were admitted to an inpatient research unit on the morning of the study. SPECT scanning was conducted in the afternoon and evening. Afterward, the bipolar disorder patients returned to the inpatient unit for 24–48 hours of observation and were discharged after their mood and vital signs returned to baseline values. Healthy subjects participated as outpatients and returned home with an escort at the end of the study.
SPECT scan
No carrier-added [
123I]IBZM was prepared by iododestannylation of the corresponding stannyl precursor as previously described
(10). [
123I]IBZM (10 mCi total dose) was administered as a bolus plus constant infusion to last for 8 hours with a bolus to infusion ratio of 3.9 hours.
Imaging was performed on the PRISM 3000 (Picker, Cleveland) camera. For each SPECT scan, three 24-minute emission images were acquired in each scan session with low-energy, high-resolution fan beam collimators (continuous mode; 128 ≥ 128 matrix; angular range, 120˚; angular step, 3˚; 40 steps; 16-cm radius of rotation). The first scan was obtained between 210 and 282 minutes after the bolus injection of radiotracer and simultaneous initiation of the constant infusion. Amphetamine was administered between 292 and 352 minutes, and the second scan was obtained between 352 and 424 minutes.
Amphetamine administration
After the first scan, subjects were taken out of the camera for intravenous administration of dextroamphetamine sulfate (0.3 mg/kg over 60 seconds) and subsequent behavioral ratings. Amphetamine administration is associated with increased activation and a need to move around; subjects felt more comfortable outside the camera. In addition, removal from the camera facilitated cardiac (ECG) monitoring and provided better access to the patient in case of significant changes in vital signs. Amphetamine was administered in the presence of a physician and a clinician, both of whom were certified in advanced cardiac life support. Previous experiments have shown that it takes approximately 60 minutes for [
123I]IBZM displacement to be achieved after amphetamine challenge
(7). Therefore, the second scan was started 60 minutes after the amphetamine injection.
Behavioral response
For all subjects, the behavioral response to amphetamine was assessed with the Brief Psychiatric Rating Scale (BPRS)
(11); Young Mania Rating Scale
(12); Amphetamine Interview Rating Scale
(13); and 10-point visual analogue scales
(5). Ratings for mood changes were obtained at 10, 30, and 50 minutes after amphetamine injection and also at the end of the second scan.
Biochemical analysis
Three blood samples were obtained during the first scan for measurement of [123I]IBZM plasma activity. Plasma activity was measured and corrected for the presence of metabolites by extraction in ethyl acetate followed by reverse-phase high-performance liquid chromatography.
Amphetamine plasma concentrations were measured by gas chromatography (SmithKline Beecham, Philadelphia) on three venous samples obtained 10, 20, and 40 minutes after amphetamine injection. The mean of the three values was used in the analysis.
Data Analysis
Images were analyzed without knowledge of the diagnosis of the subject. Images were reconstructed by using filtered back projection with a Butterworth filter of order 10 and Nyquist frequency cutoff 0.24. Individual acquisitions from both scans were coaligned and coregistered to the mean image of the first scan by using statistical parametric mapping
(14). First, a mean image was created by using the coalignment function from the three acquisitions of the first scan. Then, individual acquisitions in the second scan were coaligned with the mean image. In the last step, each acquisition from both scans was coregistered to the mean image. Uniform attenuation correction was performed within an ellipse drawn around the skull. Images were then reoriented in the canthomeatal plane. Radioactive skull markers were used for checking coregistration, identification of outline of the skull for drawing the ellipse for attenuation correction, and for reorientation.
Volumes of interest were drawn over left and right striatum (specific plus nondisplaceable uptake) and over the occipital lobe (nondisplaceable binding). Volumes of interest were placed on the mean image of the first scan by using MEDx software (Sensor Systems, Sterling, Va.). The volumes of interest were then placed in the same position on the individual acquisitions of the first and second scans.
Average counts per pixel per minute in the volumes of interest were converted to μCi/ml by using values from a calibration phantom. Specific binding was calculated as the difference between striatal and occipital binding. The ratio of specific binding to nondisplaceable uptake is proportional to receptor binding potential (B
max/K
d)
(5). This ratio was used as the primary outcome measure for radioligand binding in striatum from which we calculated the percent decrease from the first to the second scan in [
123I]IBZM binding potential that resulted from the administration of amphetamine.
Analyses were performed to compare the entire bipolar disorder group with the healthy subjects and to compare the two bipolar disorder groups (medicated and unmedicated) with the healthy subjects.
Between-group comparisons were performed with one way analysis of variance (ANOVA). Post hoc tests were conducted for any significant differences detected between groups. All tests were two-tailed.
Pearson product moment correlations were calculated for correlations between striatal [123I]IBZM binding and behavioral ratings on the Young Mania Rating Scale, BPRS, Amphetamine Interview Rating Scale, and visual analogue scales, both at baseline and at their peak after amphetamine challenge.
Results
Subjects
Fourteen euthymic bipolar patients and 16 healthy subjects agreed to take part in the study. One bipolar patient and two healthy subjects signed consent forms but withdrew before the start of the study. One healthy subject completed the first scan and the amphetamine challenge but could not complete the second scan because of nausea. The data for this subject were included in the calculation of baseline [
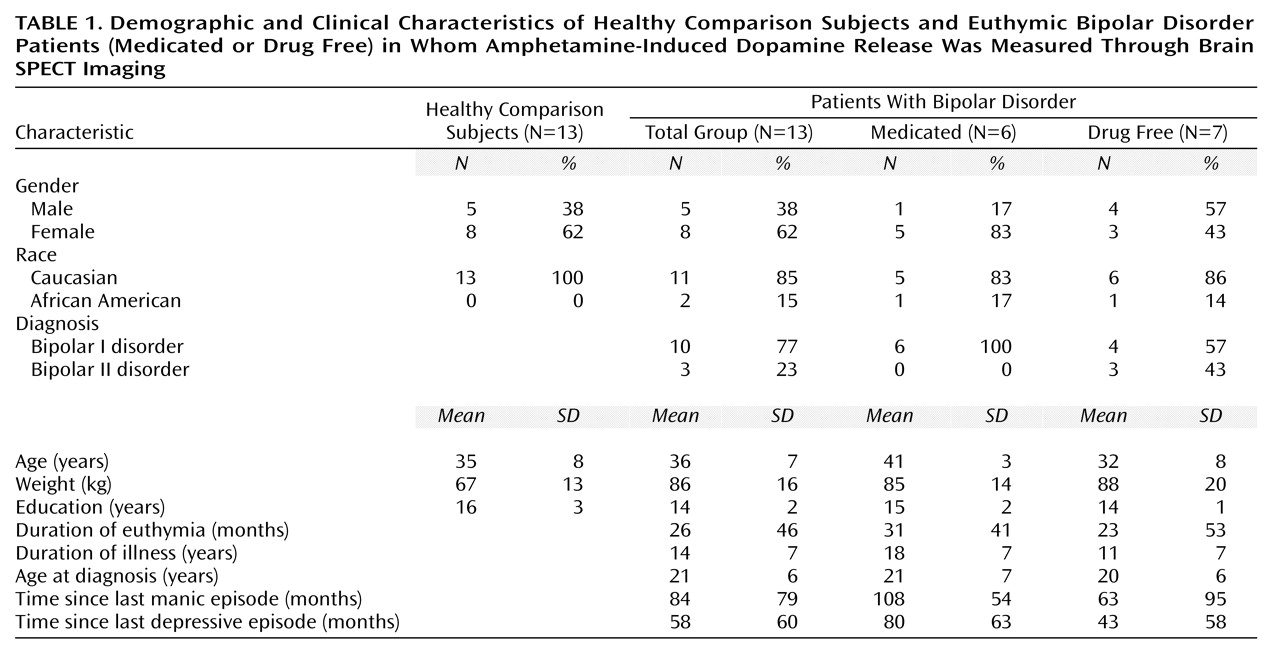
123I]IBZM binding. An equal number of 13 bipolar patients and 13 healthy subjects completed the study. Subject characteristics, including characteristics of the illness of bipolar disorder patients, are detailed in
Table 1.
Seven bipolar patients were unmedicated, and six were taking mood stabilizers (monotherapy with either lithium [N=4] or valproate [N=2]). One drug-free bipolar disorder patient had received neuroleptics 20 years earlier, whereas all others reported no history of neuroleptic exposure. Three patients with bipolar I disorder had had psychotic symptoms in the past, and one patient with bipolar II disorder had a history of psychosis that was related to substance abuse. No patient reported a history of substance abuse or dependence in the preceding 2 years. Six bipolar disorder patients reported an alcohol abuse history that occurred more than 2 years earlier (range=2–10 years). Five bipolar disorder patients reported a polysubstance abuse/dependence history that occurred more than 3 years earlier (range=3–22 years).
Scan Parameters
There were no significant differences between healthy comparison subjects and bipolar disorder patients on the total dose of [123I]IBZM injected (mean=9.78 mCi [SD=0.6] and 9.88 mCi [SD=0.4], respectively), bolus dose (mean=3.18 mCi [SD=0.2] and 3.21 mCi [SD=0.3]), time at start of the first scan (mean=215 minutes [SD=17] and 208 minutes [SD=7]), and time at start of the second scan (mean=375 minutes [SD=23] and 361 minutes [SD=12]).
Slope of percent change per hour of metabolite-corrected [123I]IBZM plasma levels was small and not different between the comparison and the bipolar disorder groups (mean=–0.05 [SD=0.34] and –0.2 [SD=0.9], respectively). Slope of percent change per hour of occipital activity from beginning of the first scan to the end of the second scan was also small and not significantly different between the two groups (mean=–0.3 [SD=1] and –0.02 [SD=1]). One-sample t tests with all subjects included showed that the distribution of slopes of change per hour of plasma levels and occipital activity were not significantly different (plasma levels: t=–0.83, df=24, p=0.41; first scan: t=–0.36, df=25, p=0.70; second scan: t=0.80, df=24, p=0.43; first to second scan: t=–1.2, df=24, p=0.24), which indicates that a steady-state plasma input function was achieved.
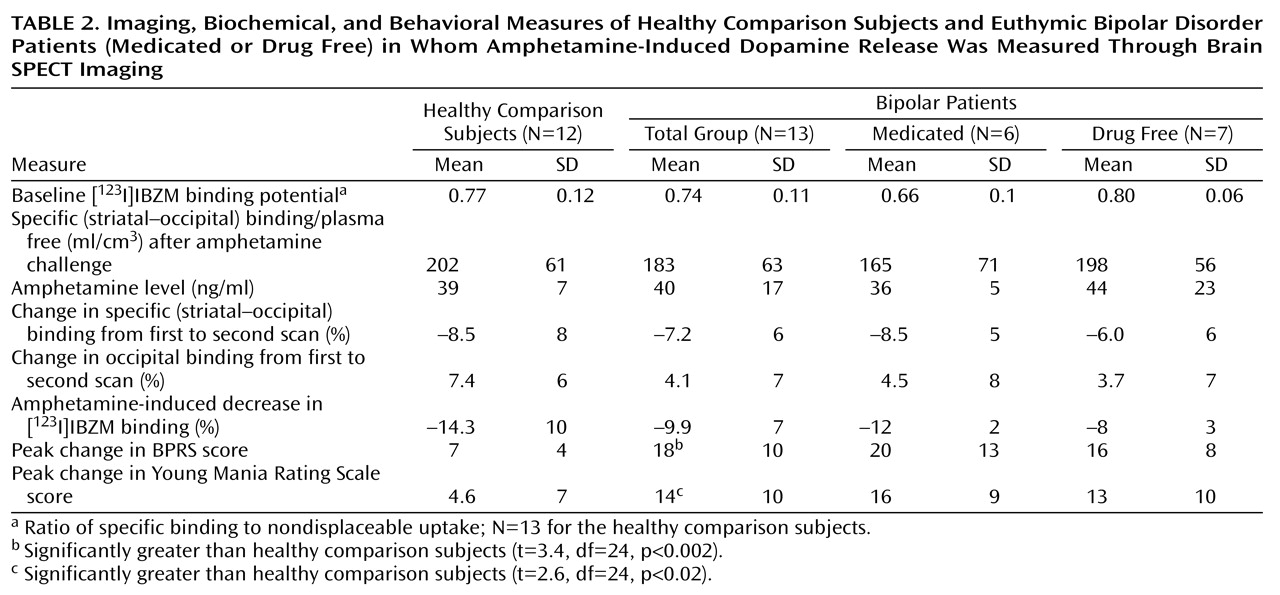
However, average occipital activity was found to be increased from the first to the second scan in all groups, although the difference between the three groups was not significant (F=0.73, df=2, 22, p=0.49) (
Table 2). These data indicated that although a steady-state input function was achieved, the nonspecific binding increased slightly after amphetamine injection and then remained steady during the second scan.
Amphetamine-Induced Changes in Pulse and Blood Pressure
The healthy comparison subjects and bipolar patients did not significantly differ in terms of peak change in systolic blood pressure (mean=53 mm Hg [SD=21] and 52 mm Hg [SD=18], respectively) (ANOVA: F=0.02, df=1, 23, p=0.87), peak change in diastolic blood pressure (mean=23 mm Hg [SD=11] and 28 mm Hg [SD=9]) (ANOVA: F=1.28, df=1, 23, p=0.27), and peak change in pulse rate (mean=7 bpm [SD=16] and 18 bpm [SD=20]) (ANOVA: F=2.0, df=1, 23, p=0.16).
Amphetamine-Induced Behavioral Changes
The healthy comparison subjects and bipolar disorder patients did not significantly differ at baseline with regard to BPRS score (mean=18 [SD=1.7] and 20 [SD=2.8], respectively) (ANOVA: F=2.22, df=1, 23, p=0.14), Young Mania Rating Scale score (mean=0.40 [SD=0.8] and 1.46 [SD=1.8]) (ANOVA: F=3.4, df=1, 23, p=0.08), and visual analogue scale ratings of anxiety (mean=2.5 [SD=1.5] and 3.3 [SD=3.4]) (ANOVA: F=0.55, df=1, 24, p=0.46). All bipolar disorder patients were judged to be stable and clinically euthymic at baseline.
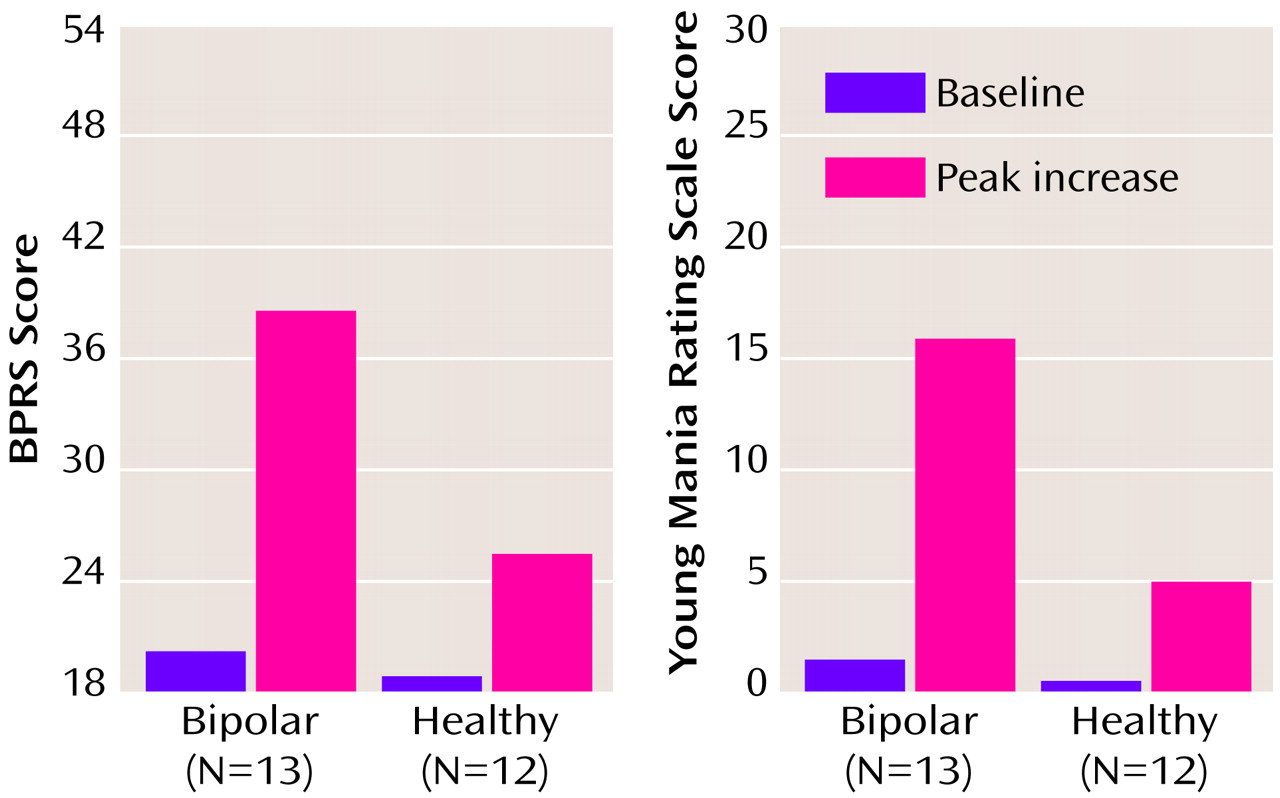
Amphetamine administration led to a substantial increase in behavioral symptoms as measured by the BPRS and Young Mania Rating Scale in both the healthy and bipolar groups (
Figure 1). However, the peak behavioral response was significantly greater in the bipolar than in the healthy group on both the Young Mania Rating Scale and BPRS ratings (
Table 2). The increase in behavioral ratings did not significantly differ between the medicated and drug-free bipolar disorder patients: BPRS (ANOVA: F=0.60, df=1, 11, p=0.60); Young Mania Rating Scale (ANOVA: F=0.38, df=1, 11, p=0.60).
The increase in behavioral response was transient, and most subjects were back to baseline at the end of the second scan. None of the bipolar disorder patients had a sustained mood elevation, and all were back to baseline mood state within 24 hours of the last amphetamine dose.
Baseline [123I]IBZM Binding Potential
As seen in
Table 2, the healthy comparison subjects and bipolar disorder patients did not significantly differ at baseline in terms of [
123I]IBZM binding potential (one-way ANOVA: F=0.94, df=1, 24, p=0.34). The [
123I]IBZM binding potential of the medicated bipolar disorder subjects was decreased relative to that of the healthy comparison subjects and the drug-free bipolar patients, but this difference did not reach statistical significance (ANOVA: F=3.2, df=2, 23, p=0.06). However, medicated bipolar disorder subjects were older than the nonmedicated bipolar disorder subjects, and when age was added as a covariate, the difference between the three groups diminished (analysis of covariance: F=2.07, df=2, 20, p=0.15).
Amphetamine Effect on [123I]IBZM Binding Potential
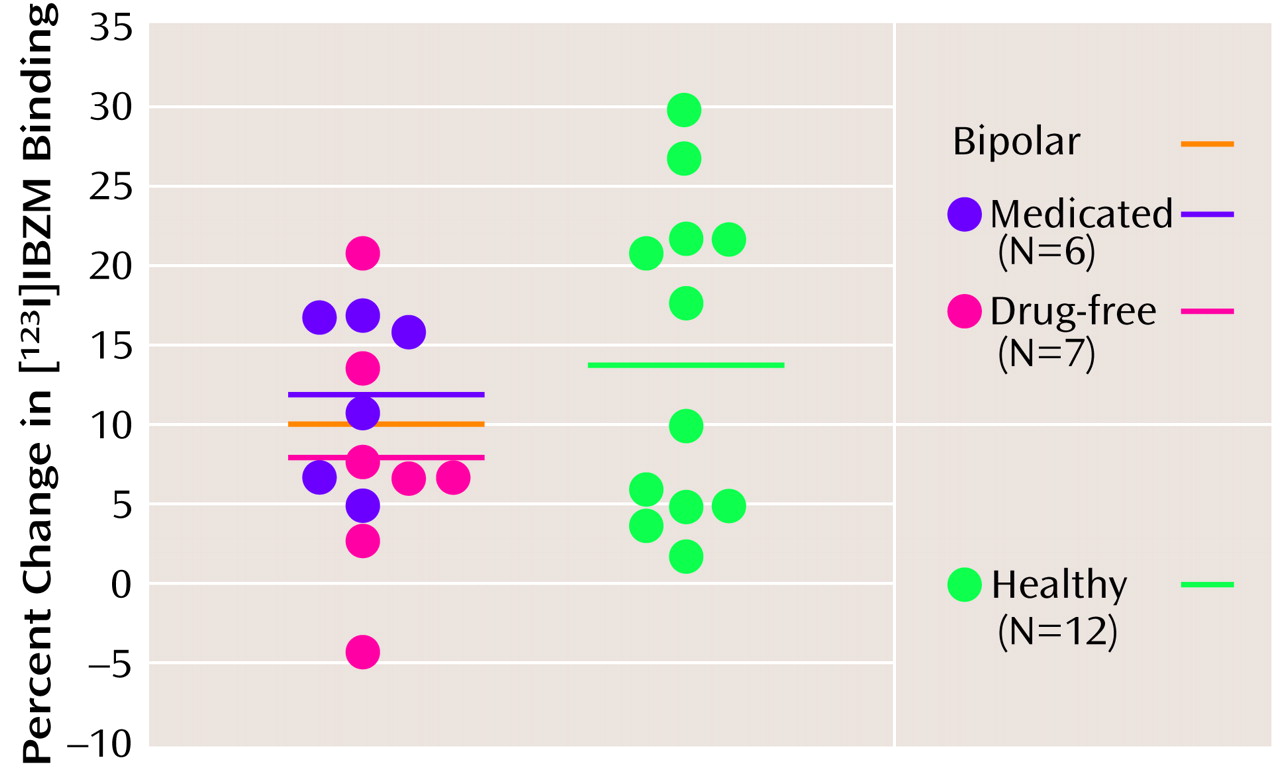
Amphetamine-induced decrease in [
123I]IBZM binding potential for the healthy comparison subjects did not significantly differ from that of the bipolar subjects, either as a group (ANOVA: F=1.7, df=1, 23, p=0.20) or by medication status (ANOVA: F=1.2, df=2, 22, p=0.31) (
Figure 2,
Table 2). The variance of the amphetamine effect on [
123I]IBZM binding potential was not significantly different between the healthy and bipolar disorder groups (variance ratio=0.47, F=4.76, df=1, 23, p=0.22).
Women (N=15) had a greater decrease in [123I]IBZM binding potential than men (N=10) (mean=–14.9% [SD=8%] versus –7.7% [SD=8%], respectively) (ANOVA: F=4.9, df=1, 23, p=0.03). However, when two female outliers in the healthy group (decreases of 24% and 30%) were excluded, the difference between men and women was not significant. The amphetamine-induced decrease in [123I]IBZM binding potential was less in the six patients with a history of polysubstance abuse (mean=–6%, SD=7%) than in the seven patients without such a history (mean=–13%, SD=5%), but the difference was not statistically significant (ANOVA: F=4.6, df=1, 11, p=0.06). Amphetamine-induced decrease in [123I]IBZM binding potential did not correlate with amphetamine levels, change in systolic and diastolic blood pressure, age, or weight of subjects. A significant correlation was present between age and baseline [123I]IBZM binding potential (r=0.82, df=24, p<0.001).
Correlation Between Behavioral Response and [123I]IBZM Binding
An exploratory analysis was performed to assess correlations between imaging results and behavioral measures. Although none of these correlations were significant after Bonferroni correction, this exploration was initiated to help guide future studies.
In bipolar subjects, amphetamine-induced decrease in [123I]IBZM binding did not correlate with peak postamphetamine scores on the Young Mania Rating Scale (all bipolar disorder subjects: r=0.00, df=11, p=0.99; medicated bipolar subjects: r=0.36, df=4, p=0.50; drug-free bipolar subjects: r=–0.09, df=5, p=0.85); BPRS (total group: r=0.17, df=11, p=0.58; medicated: r=0.18, df=4, p=0.75; drug free: r=0.40, df=5; p=0.40); or visual analogue scale ratings of anxiety (total group: r=0.40, df=11, p=0.17; medicated: r=0.70, df=4, p=0.13; drug free: r=0.31, df=5, p=0.51).
In healthy subjects, there were positive (but not statistically significant) correlations between amphetamine-induced decrease in [123I]IBZM binding and increases in scores on the Young Mania Rating Scale (r=0.55, df=9, p=0.07), BPRS (r=0.58, df=9, p=0.07), and visual analogue scale ratings of anxiety (r=0.42, df=10, p=0.20).
Factorial analysis of the 10-point visual analogue scale ratings identified items that comprised a euphoria factor (rush, high, good, powerful, pleasure, happy, energetic, and restlessness) as well as items that comprised a dysphoria factor (bad, paranoid, and anxiety). Factorial analysis of ratings on the Amphetamine Interview Rating Scale identified items that comprised a motor factor (anxiety, feeling energetic, and restlessness). Positive correlations were seen between amphetamine-induced decrease in [123I]IBZM binding and peak postamphetamine motor factor scores for all subjects (r=0.44, df=23, p=0.03), healthy subjects (r=0.63, df=10, p=0.03), and all bipolar disorder subjects (r=0.55, df=11, p=0.05).
Negative correlations were seen between baseline [
123I]IBZM binding potential and peak postamphetamine euphoria factor scores for all subjects (r=–0.42, df=24, p=0.04) and for the drug-free bipolar disorder group (r=–0.84, df=5, p=0.02), a finding that has also been reported in other studies
(15).
Discussion
The findings from this study show that a greater behavioral response to an amphetamine challenge in euthymic bipolar disorder patients (both drug free and medicated) was not accompanied by a significant difference in decrease in [
123I]IBZM binding between the two groups. Since a decrease in [
123I]IBZM binding has been shown to be related to amount of striatal dopamine release, the results of the study indicate that mania-like symptoms induced by amphetamine may not be related to increased dopamine release. Instead, these data are more consistent with an increased postsynaptic dopamine response in bipolar disorder. The findings of our study also indicate no differences in baseline D
2 receptor binding between healthy comparison subjects and bipolar disorder patients. Pearlson and colleagues have previously reported no differences in D
2 receptor binding between healthy subjects and bipolar disorder patients with nonpsychotic mania
(9). Together these findings suggest that vulnerability to mania in patients with bipolar disorder does not seem to be due to increased striatal D
2 receptors.
A limitation of this study was the small sample size. However, the difference between the groups in amphetamine-induced decrease in [123I]IBZM binding was also small, and power analysis calculations indicated that a study group size of approximately 300 subjects would be required to show a significant difference between healthy and bipolar patients, if, in fact, such a difference existed.
The bipolar subjects in this study were euthymic. It is possible that a state of increased dopamine release may be present during a manic episode. Amphetamine-induced decrease in striatal [
123I]IBZM binding has been shown to correlate with baseline state of activation in acutely ill schizophrenic subjects
(16), and the same may by true for mania
(16). In the current study, euthymic bipolar disorder patients had a greater behavioral response to amphetamine than healthy subjects did. Amphetamine-induced activation and euphoria has been used as a model for mania
(1,
3,
13). Therefore, even though bipolar disorder patients were initially euthymic, a transient hypomania was induced with amphetamine administration. Despite a greater behavioral response in bipolar disorder patients (i.e., hypomania), a greater decrease in [
123I]IBZM binding was not seen nor did the decrease correlate with Young Mania Rating Scale scores. These observations suggest that the greater amphetamine-induced behavioral response seen in bipolar disorder patients could not be attributed to increased dopamine release.
There were no significant differences seen between medicated and unmedicated bipolar disorder patients on the variables measured in this study. Unmedicated bipolar disorder patients, however, showed a lowered decrease in [
123I]IBZM binding potential in response to amphetamine than medicated bipolar disorder patients. The effect of mood stabilizer medication would seem to be to increase dopamine release to a level similar to that seen in healthy subjects. Lithium has been reported to increase tyrosine hydroxylase activity and thereby increase catecholamine turnover
(17). This observation needs further study.
The increased occipital activity from the first to the second scan could have several causes. [
123I]IBZM excretion or metabolism could be decreased due to changes in blood pressure and vasoconstriction in clearance organs, such as the liver and kidney. Amphetamine could have changed global and regional cerebral blood flow; however, [
123I]IBZM binding is relatively immune to changes in blood flow when the bolus and constant infusion method is used
(5). Alternatively, incomplete coregistration between the two scans could lead to some changes in measurement of occipital activity. The difference in occipital activity before and after amphetamine injection was greater in the healthy group than in the bipolar group. There was a greater increase of occipital activity from the first to the second scan in the healthy subject group (
Table 2). Therefore, amphetamine-induced decrease in [
123I]IBZM binding could have been overestimated in this group. Since this decrease was greater in the healthy subject group, the patients and healthy subjects were probably even more similar with respect to amphetamine-induced decrease in [
123I]IBZM binding than the reported values, which suggests no difference between the two groups in terms of amphetamine-stimulated dopamine release.
In this study we measured striatal dopamine release. Changes in cortical regions (e.g., the limbic system) may be different from what is seen in the striatum. Even within the striatum, the SPECT scan is unable to distinguish the ventral striatum, which is part of the limbic system and is more likely to show changes with changes in emotional states. To study cortical changes, this experiment will have to be repeated with a cortical dopamine tracer. PET scan techniques can provide the higher resolution needed to distinguish the ventral from the dorsal striatum.
A limitation of this study is that it did not measure changes in the norepinephrine system. Even though evidence from some studies suggests a greater role of dopamine in mania
(1), amphetamine increases norepinephrine as well as dopamine release. The arousal and euphoric effects of amphetamine may be related to changes in the norepinephrine release, and therefore this study did not detect any changes in the dopamine system. This hypothesis can be tested with radiotracers that are specific for noradrenergic receptors. However, there is no evidence to suggest that amphetamine effects on norepinephrine and the dopamine system are dissociated from each other in parts of the brain. Even if the euphoric effects were norepinephrine mediated, one would expect to see changes in the dopamine system as well. It is also possible that changes in another abnormal neurotransmitter system such as the serotonergic system or glutamatergic system may have been triggered by amphetamine-induced catecholamine release.
Keeping the above caveats in mind, the greater behavioral response of bipolar patients to amphetamine without overt evidence of increased striatal dopamine or increased number of postsynaptic dopamine receptors suggests that postsynaptic mechanisms, including the receptor itself or subsequent signal transduction pathways, may be responsible. Consistent with this interpretation, a number of studies have suggested postsynaptic signal transduction abnormalities in bipolar disorder. Postmortem studies have indicated enhanced cAMP, G protein, and phosphokinase levels activity in bipolar disorder
(18,
19). Furthermore, lithium and other mood stabilizers have been shown to mediate their effects through actions on the phosphatidylinositol and protein phosphokinase intracellular signal transduction systems
(20). Therefore, further studies should be undertaken to identify postsynaptic abnormalities that may be present in bipolar disorder.