Recently, Reynaud et al.
(1) reported that the combination of buprenorphine and flunitrazepam resulted in unexpected deaths. A possible mechanism underlying such an interaction would be inhibition of buprenorphine metabolism by flunitrazepam or vice versa. Since buprenorphine and flunitrazepam both undergo
N-dealkylation by the drug-metabolizing cytochrome P450 enzyme 3A4, it is possible that concomitant use of the two drugs could result in higher than expected concentrations of either (or both) of the drugs because of mutual competitive inhibition.
Buprenorphine is an opioid derivative of the morphine alkaloid thebaine, used in the treatment of opiate addiction. In humans, the principal route of buprenorphine metabolism is by means of
N-dealkylation of the
N-cyclopropylmethyl group to norbuprenorphine by cytochrome P450 3A4 (CYP3A4)
(2,
3).
Flunitrazepam is a benzodiazepine derivative that is primarily used as a sedative and hypnotic
(4). The major metabolites for flunitrazepam in humans are desmethylflunitrazepam, 3-hydroxyflunitrazepam, and 7-aminoflunitrazepam
(5,
6). We have identified the major enzymes that mediate the formation of these metabolites as CYP3A4 and the polymorphically expressed CYP2C19
(7). CYP3A4 mediates 80% of the formation of 3-hydroxyflunitrazepam and approximately 40% of desmethylflunitrazepam formation. CYP2C19 is the major enzyme that catalyzes desmethylflunitrazepam formation by contributing to 60% of total metabolite formation.
The aim of the current study was to determine if buprenorphine and flunitrazepam are likely to interact, resulting in higher plasma concentrations of either one or both drugs when taken together.
Method
Experiments were carried out in three human liver microsome preparations carrying the CYP2C19*1/*1 allele. The cDNA-expressed CYP2C19 and CYP3A4 microsomes expressed in a baculovirus/insect cell expression system were purchased from Gentest Co. (Woburn, Mass.). Ultraviolet high-performance liquid chromatography assays established for investigating the metabolism of flunitrazepam and omeprazole in vitro were used to quantitate the effect of buprenorphine on the metabolism of both drugs.
Chemical inhibition of flunitrazepam metabolism with varying concentrations of buprenorphine and norbuprenorphine in human liver microsomes and cDNA-expressed human CYP2C19 and CYP3A4 microsomes were carried out. As a positive control inhibition of omeprazole, metabolism to 5-hydroxyomeprazole (CYP2C19) and omeprazole sulfone (CYP3A4) by buprenorphine and norbuprenorphine were also carried out in the same series of microsomes.
Inhibition experiments were carried out by using a range of buprenorphine (0.1 and 20 μM) and norbuprenorphine (10 and 100 μM) levels that is higher than the range of levels achieved in the plasma (0.002–1.0 μM). Typical clinical plasma concentrations of buprenorphine exhibited no inhibition of flunitrazepam.
Inhibition of either flunitrazepam or omeprazole metabolism in human liver microsomes was carried out at substrate concentrations equal to the K
m of the metabolite of interest. Concentrations of buprenorphine used in subsequent K
i determinations (5, 10, 20, 40 μM) were selected on the basis of these experiments. The type of inhibition and apparent K
i (inhibitor constant) values were determined by using the graphical methods of Dixon
(8) and Cornish-Bowden
(9).
The predicted in vivo inhibition of the catalytic activity of CYP3A4 in the metabolism of flunitrazepam by buprenorphine was calculated as follows
(10): % inhibition=[I]/([I]+k
i)×100. Concentrations of buprenorphine in vivo range from 2.1 to 35 nM in plasma and organs
(1). These values were used as the inhibitor concentration
[I]. K
i values entered into this formula were those which were measured in this study.
The in vivo inhibition of buprenorphine N-dealkylation by flunitrazepam was also estimated. Ki values for this reaction were assumed to be equal to the Km for the formation of 3-hydroxyflunitrazepam formation (CYP3A4-mediated reaction).
Results
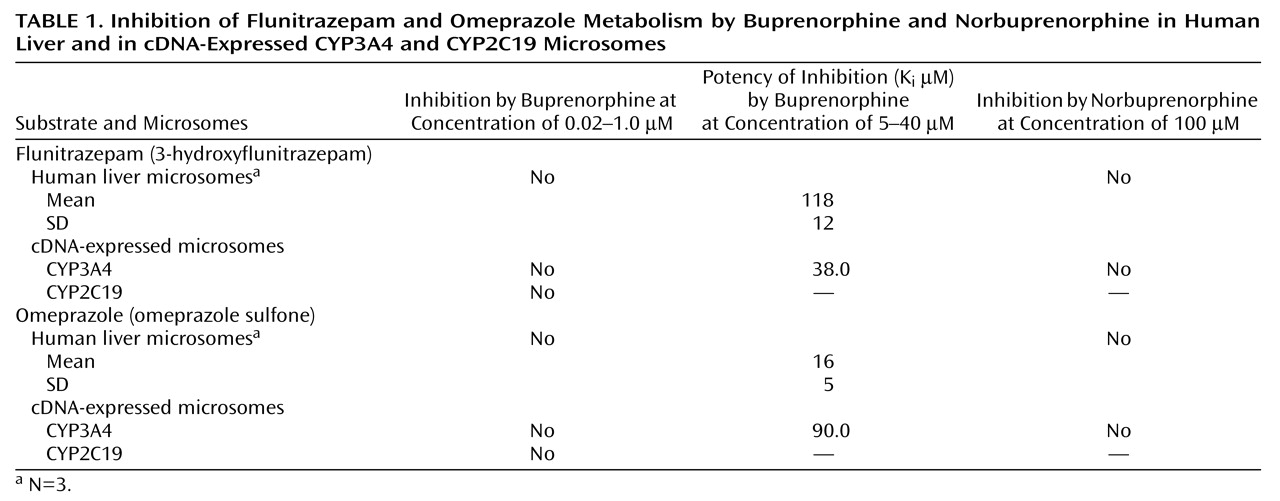
The results are summarized in
Table 1. Buprenorphine competitively inhibited CYP3A4-mediated metabolism of flunitrazepam, but only at high concentrations. Differences in K
i between the cDNA-expressed and human liver microsomes are likely due to differences in conformation in their membranes. The cDNA-expressed CYP enzymes from the baculovirus/insect cell expression systems typically have higher catalytic turnover rates, which may make them more susceptible to inhibition.
CYP2C19-mediated formation of desmethylflunitrazepam and 5-hydroxyomeprazole was not inhibited by buprenorphine, which suggests that CYP2C19 does not metabolize buprenorphine. Norbuprenorphine did not inhibit either flunitrazepam or omeprazole metabolism, suggesting that this metabolite does not interact with CYP2C19 and CYP3A4.
Discussion
Most drugs circulate in the plasma at concentrations much less than the concentrations resulting in one-half the maximal rate of metabolism (K
m). For example, buprenorphine concentrations in plasma after a single sublingual administration of a 0.4-mg dose are typically between 0.45 and 0.84 ng/ml (8.5×10
–4–1.71×10
–3 μM) (bound plus free), and the K
m for the
N-dealkylation of buprenorphine is 39.3 μM
(2,
11). Equivalent total concentrations and K
m of flunitrazepam following a 2.0-mg oral dose are about 2.5 ng/ml (5.34×10
–3 μM) and 40.0 μM, respectively, for the
N-dealkylation reaction. We adjusted our in vitro conditions to reflect the clinical situations and then estimated the impact in vivo from our in-vitro-derived calculation data.
Based on these values, the projected in vivo inhibition of CYP3A4-mediated metabolism of flunitrazepam by buprenorphine is only between 0.1% and 2.5% at therapeutic and higher-than-therapeutic plasma concentrations of buprenorphine. As well, the estimated in vivo inhibition of buprenorphine N-dealkylation by flunitrazepam at typical plasma concentrations of flunitrazepam is a miniscule 0.08%. If the high suffix of protein binding of each drug is also taken into account, the extent of interaction is likely further reduced because the free drug concentration is even lower.
These data indicate that concomitant administration of either drug will have little effect on the plasma concentrations of the other drug. Therefore, deaths in drug abusers taking a combination of the drugs must be pharmacodynamic. Both buprenorphine and flunitrazepam can depress respiration; therefore, an interaction is not surprising.
In conclusion, our in vitro data do not support a buprenorphine-flunitrazepam metabolic interaction at the concentrations that occur in humans after concomitant administration of both drugs.