Depression is associated with higher than normal rates of mortality in patients with ischemic heart disease
(1). This association may be due in part to the increased platelet activation seen in depressed subjects both with and without ischemic heart disease or risk factors for ischemic heart disease
(2–
6). The mechanism by which platelet activation is increased in depression is unknown. One possibility involves the serotonin system, as both platelets and brain neurons share the same serotonin transporter gene and protein and the same serotonin-transporter-linked promoter region (5-HTTLPR). The expression of the serotonin transporter gene is affected by the 5-HTTLPR polymorphism; the dominant
s allele of the 5-HTTLPR polymorphism is associated with the production of fewer serotonin transporters, whereas the recessive
l allele is associated with the production of a greater number of serotonin transporters
(7). Variations in the 5-HTTLPR polymorphism have been associated with mood disorders
(8). However, it is not known whether the 5-HTTLPR polymorphism influences platelet activation.
We investigated the relationship among depression, platelet activation, and 5-HTTLPR genotype in a group of elderly subjects without overt ischemic heart disease. We hypothesized that subjects with the l/l genotype would demonstrate greater platelet activation as their platelets should store more serotonin in their dense granules and therefore should release more serotonin and activate more platelets in response to subclinical vascular damage.
Method
From 96 participants in a study of pharmacotherapy of late-life depression
(9), 61 elderly depressed subjects were recruited. The subjects included in this study were age 60 years or older, met the DSM-IV criteria for a major depressive episode without psychotic features, and had a baseline score on the Hamilton Depression Rating Scale
(10) of 15 or higher, a Mini-Mental Status Examination (MMSE)
(11) score of 15 or higher, and no current history of substance abuse. Two indices of platelet activation—platelet factor 4 and β-thromboglobulin—were measured as previously described
(5). Cardiovascular disease burden was estimated by summing the heart and vascular disease subscales of the Cumulative Illness Rating Scale
(12). Genotyping for the 5-HTTLPR polymorphism was performed as previously described
(13) with 56 of the depressed subjects. Twelve nondepressed elderly subjects were recruited to form the comparison group and were assessed in the same manner as for the depressed subjects but were not genotyped. Written informed consent was obtained from all subjects.
We used t tests and Fisher’s exact tests to contrast the depressed subjects with the comparison group and an analysis of covariance (ANCOVA) to compare the platelet factor 4 concentrations while controlling for MMSE score and age. We calculated a Cohen’s r to measure effect size. In the analysis based on genotype, we used t tests and Fisher’s exact tests with log transformations of platelet factor 4 and β-thromboglobulin levels (to help normalize the distribution), an ANCOVA while controlling for Hamilton depression scale score, and a Cohen’s r to measure effect size.
Results
The depressed subjects were significantly older (mean age=72.4 years, SD=7.8) than the comparison subjects (mean age=67.3, SD=5.4) (t=–2.15, df=71, p=0.04) and more cognitively impaired, according to MMSE scores (mean=26.8, SD=3.3, versus mean=29.1, SD=1.2) (t=4.18, df=50.5, p=0.0001), but they did not differ from the comparison group in percentage of male subjects (26.2% [N=16] versus 33.3% [N=4]) (p=0.73, Fisher’s exact test) or percentage who smoked (15.3% [N=9] versus 0%) (p=0.34, Fisher’s exact test; data missing for two depressed subjects). The mean scores on the Cumulative Illness Rating Scale for heart and vascular disease were low in both groups, indicating minimal cardiovascular disease burden (mean=1.7, SD=1.5, versus mean=1.3, SD=1.3) (t=–1.07, df=71, p=0.29). Platelet factor 4 and β-thromboglobulin levels were significantly higher in the depressed subjects than in the comparison group; the mean platelet factor 4 levels were 34.7 IU/ml (SD=28.4) and 12.7 IU/ml (SD=22.0), respectively (t=–4.30, df=71, p<0.0001), and the mean β-thromboglobulin levels were 119.4 IU/ml (SD=103.1) and 50.1 IU/ml (SD=80.6) (t=–2.95, df=49, p=0.005). The differences remained significant even after we controlled for age (F=15.71, df=1, 70, p=0.0002; Cohen’s r=0.43) and MMSE score (F=15.38, df=1, 70, p=0.0002; Cohen’s r=0.42).
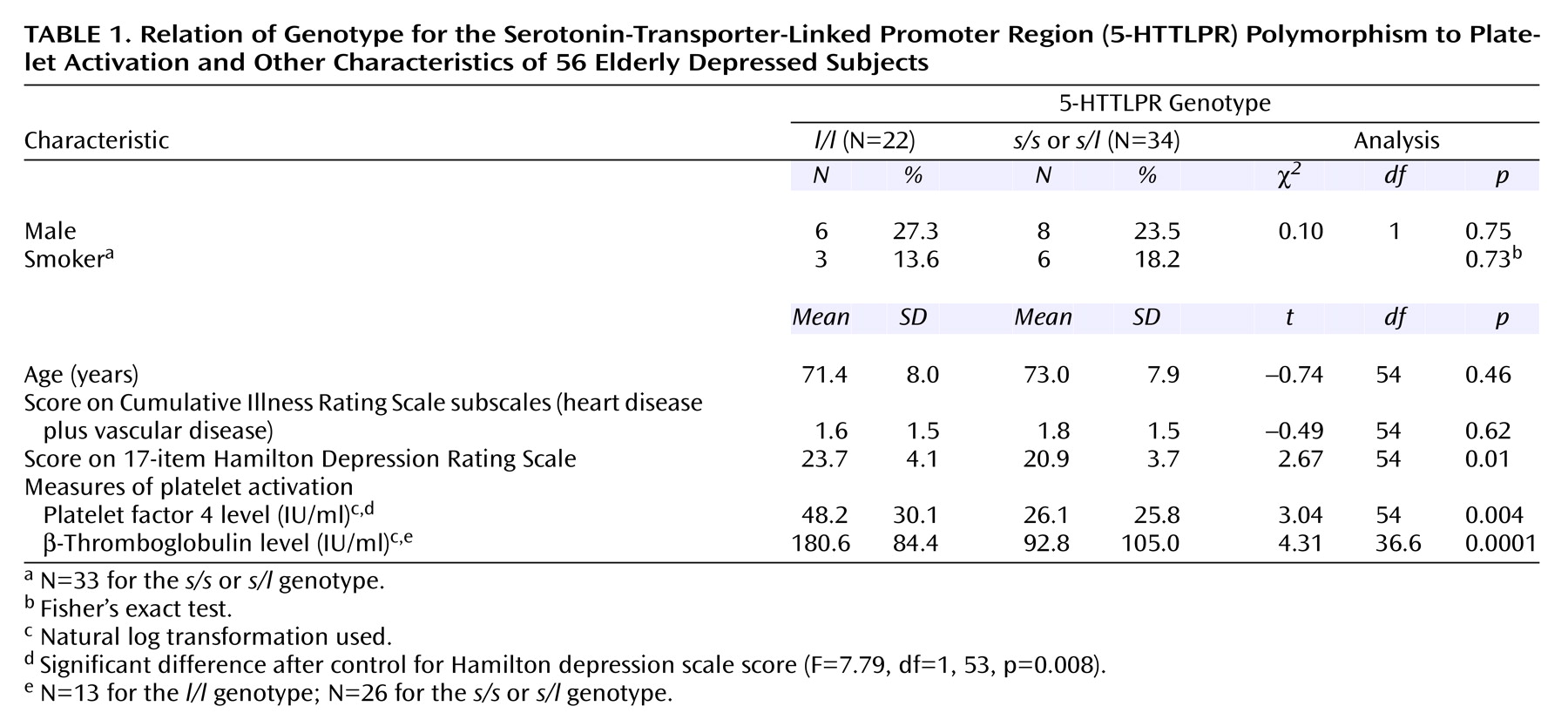
In the 56 depressed subjects classified by 5-HTTLPR polymorphism (
Table 1), platelet factor 4 and β-thromboglobulin levels were highly correlated (r=0.75, p<0.0001, N=39). Depressed subjects with the
l/l genotype had significantly higher Hamilton depression scale scores but were otherwise clinically similar to the other depressed subjects. The depressed subjects with the
l/l genotype had significantly higher platelet factor 4 and β-thromboglobulin levels. The difference in platelet factor 4 remained significant after we controlled for the difference in Hamilton depression scale score; effect size was indicated by Cohen’s r=0.36.
Discussion
In our group of elderly subjects with low cardiovascular disease burden, major depression was associated with higher levels of platelet factor 4 and β-thromboglobulin. Depressed subjects who were homozygous for the l/l 5-HTTLPR allele had more severe depression and demonstrated the greatest elevation in platelet factor 4 and β-thromboglobulin.
These findings replicate those of studies demonstrating greater platelet activation in depressed subjects than in nondepressed comparison subjects
(2–
6), and we believe it is the first to demonstrate greater than normal platelet activation in depressed elderly subjects with low cardiovascular disease burden. To our knowledge, this is also the first study of the association between 5-HTTLPR polymorphism and platelet activation in depression. Our finding supports our hypothesis that platelets in persons with the
l/l genotype are more efficient in the uptake
(14) and storage of serotonin in their dense granules. Thus, one could imagine that when a platelet in an
l/l carrier becomes activated by “bumping” into a subclinical atherosclerotic plaque
(14), the activated platelet releases more serotonin and therefore activates a larger number of circulating platelets than in someone without the
l/l genotype. This greater activation could lead to greater thrombus formation, which may be more likely to result in an adverse cardiovascular event, such as a myocardial infarction. Our finding is particularly interesting in light of the higher rate of mortality associated with depression in patients with ischemic heart disease
(1); the observed increase in mortality might be accounted for by greater platelet activation in depressed individuals with the
l/l 5-HTTLPR genotype. If this finding is replicated, genotyping could be used to help identify the patients with ischemic heart disease at greatest risk of depression-associated cardiovascular mortality and thus allow for the testing of focused research intervention(s). It should be noted, however, that our hypothesis regarding increased uptake and storing of serotonin conflicts with some evidence that the 5-HTTLPR genotype is not associated with platelet serotonin concentration
(15).
The strengths of this study include the rigorous psychiatric and medical assessment of the subjects and the use of a group of subjects with minimal cardiovascular disease. The main limitation is the relatively small number of subjects and the use of a sample of convenience.
While this novel finding does not have immediate clinical implications, it does illustrate the potential influence of genetic differences on illness course and treatment outcome. Future studies are needed to replicate this association between the l/l 5-HTTLPR genotype and increased platelet activation in geriatric depression; to clarify the relationship among 5-HTTLPR polymorphism, depression, ischemic heart disease, and platelet activation in community-dwelling subjects; and to investigate the association of the l/l genotype with cardiovascular events in depressed patients with ischemic heart disease.