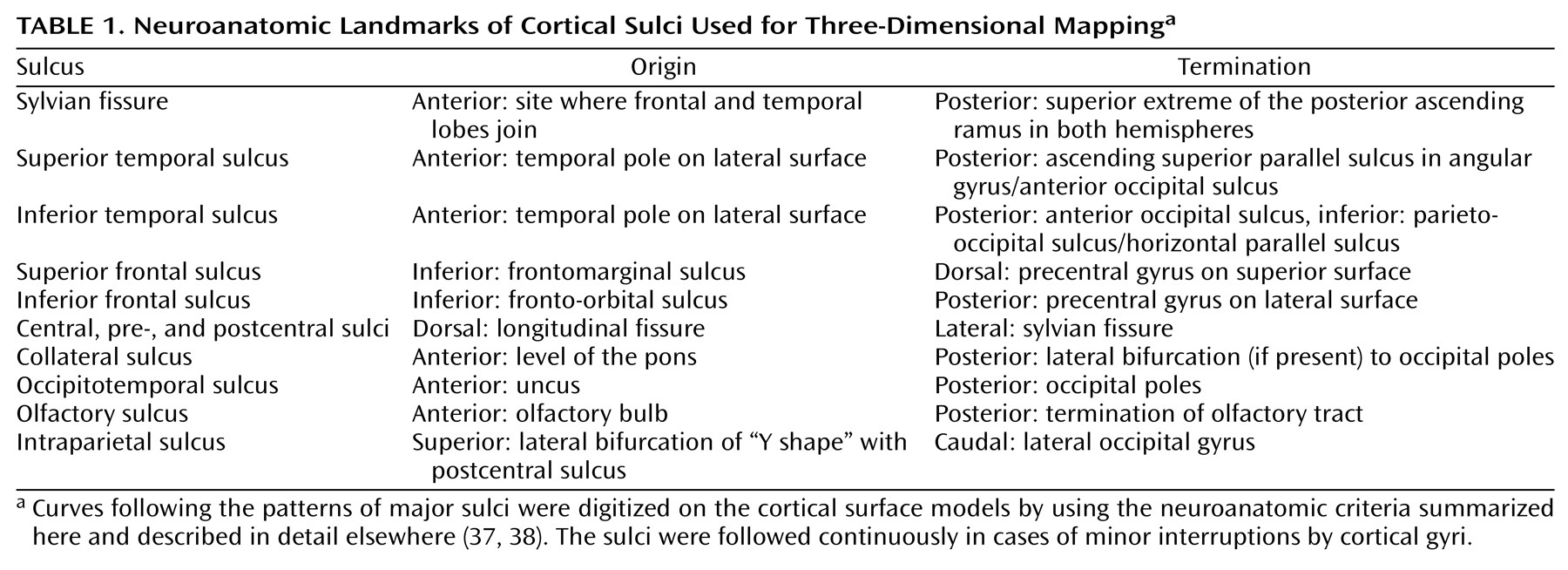
Disturbances in structural brain asymmetries in schizophrenia have been reported, although some empirical data contradict this general hypothesis
(2,
3,
18,
44,
45). The majority of evidence suggests, however, that the pathophysiological mechanisms are diffuse, involving structural and functional systems in both hemispheres, although the left hemisphere is more widely implicated
(5). At the cortex, gyral asymmetries have been shown as undisturbed, less lateralized compared to normal, and even reversed in schizophrenia (e.g., references
2,
3,
8,
9,
15,
18,
46).
Cortical Asymmetries
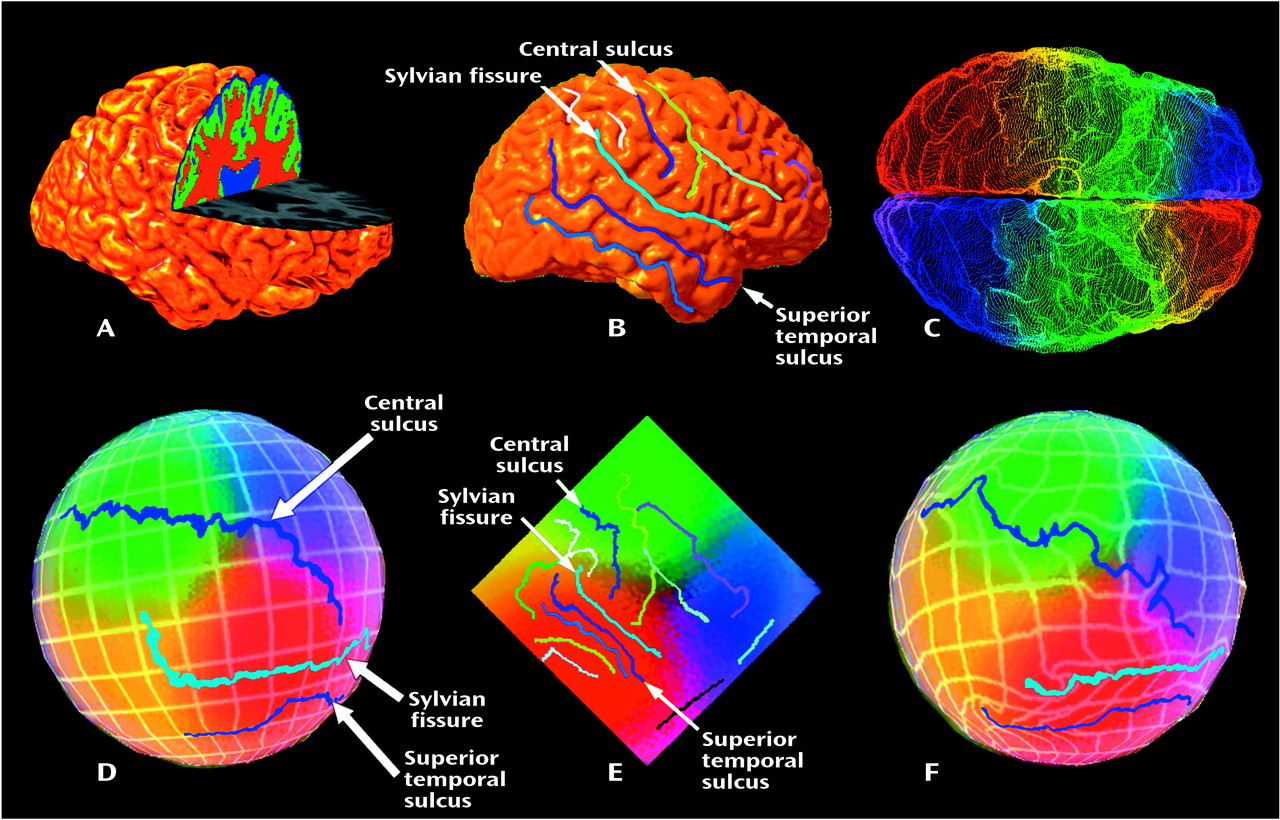
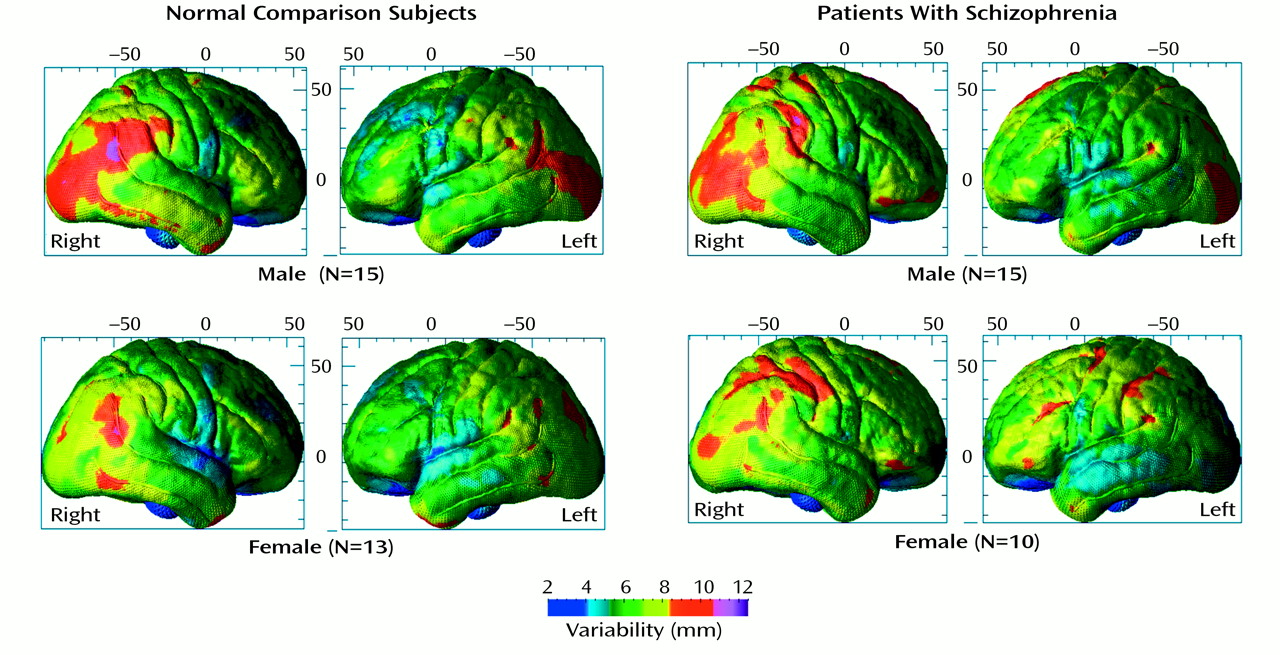
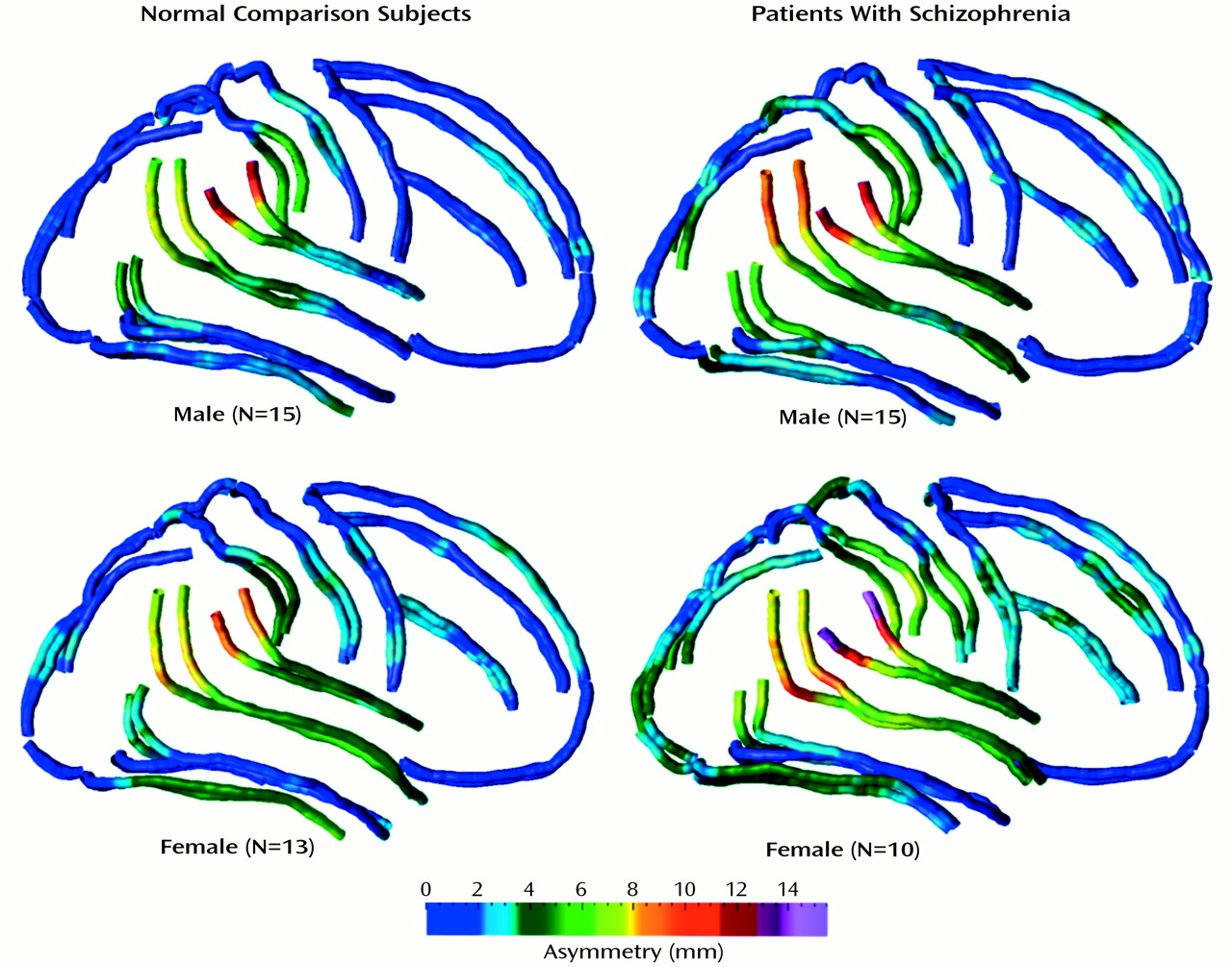
We mapped sulcal asymmetries across the entire cortical surface in schizophrenic and comparison subjects. Statistical analyses confirmed the presence of significant sulcal asymmetries in temporoparietal regions in both diagnostic groups (
Figure 5). Several of these asymmetries are well established in normal populations, but to our knowledge, they have not been previously mapped in three dimensions. For example, it is known that the sylvian fissure extends further posteriorly in the left hemisphere (corresponding to greater planum temporale asymmetries in these regions) and rises more steeply in the right hemisphere (e.g., references
27,
47,
48). Our results are similar, showing greater than normal left hemisphere sylvian fissure length and right hemisphere slope and height (
Figure 6). These asymmetries were largely mirrored in the superior and inferior temporal sulci.
Postcentral gyrus hemispheric asymmetries have been reported in normal adults
(49) and in Alzheimer’s disease patients (right-handed men)
(29). Our results corroborate these findings, revealing significant asymmetry of the postcentral sulcal anterior extreme (right hemisphere greater than left hemisphere) in both diagnostic groups. Such asymmetry may be associated with asymmetries of the slope and horizontal length of the sylvian fissure and temporal sulci and may reflect asymmetries in the parietal operculum that complement planum temporale asymmetries (not necessarily related) in right-handed subjects
(50).
Many earlier studies assessing perisylvian asymmetries in patients with schizophrenia focused on the planum temporale, a triangular region on the superior temporal gyral surface that is involved in language functions. The findings indicate abnormally low planum temporale volume and area in the left hemisphere of patients with schizophrenia (e.g., references
15 and
51). Negative findings exist, however, and discrepancies in results are difficult to interpret given the differences in the measurement techniques used
(2,
18). Although sylvian fissure measurements and planum temporale area/volume asymmetries overlap, they should be considered different measures
(52–
54). That is, typically the planum is found to be symmetrical when the boundaries include the posterior ascending ramus of the sylvian fissure
(55). This appears to indicate, as others have suggested, that the horizontal segment is a better indicator of planum temporale asymmetry
(52) and that the right hemisphere ascending segment compensates for the shorter horizontal segment. Cytoarchitectonically, however, the auditory association cortices extend into the ascending part of the sulcus
(55).
In this study, the sylvian fissure was not divided at the cortex to specifically isolate planum temporale regions, and no conclusions regarding abnormalities of laterality of the planum in schizophrenia are assumed. In previous studies, both the sylvian fissure and the anatomically overlapping planum temporale showed large hemispheric asymmetry effects in normal subjects. According to Cohen
(56,
57), an effect size, f, is considered small if f is 0.10, medium if f is 0.25, and large if f is beyond 0.40. As in the earlier studies, we found hemispheric asymmetry effects with f values greater than 0.40. Since studies with relatively small study groups have shown hemispheric asymmetries in schizophrenia that are less than in healthy subjects, and since some studies have even shown complete reversals of hemispheric asymmetries
(15), effect sizes for diagnosis-by-hemisphere interactions for sylvian fissure measurements might similarly be expected to be large or at least medium in magnitude.
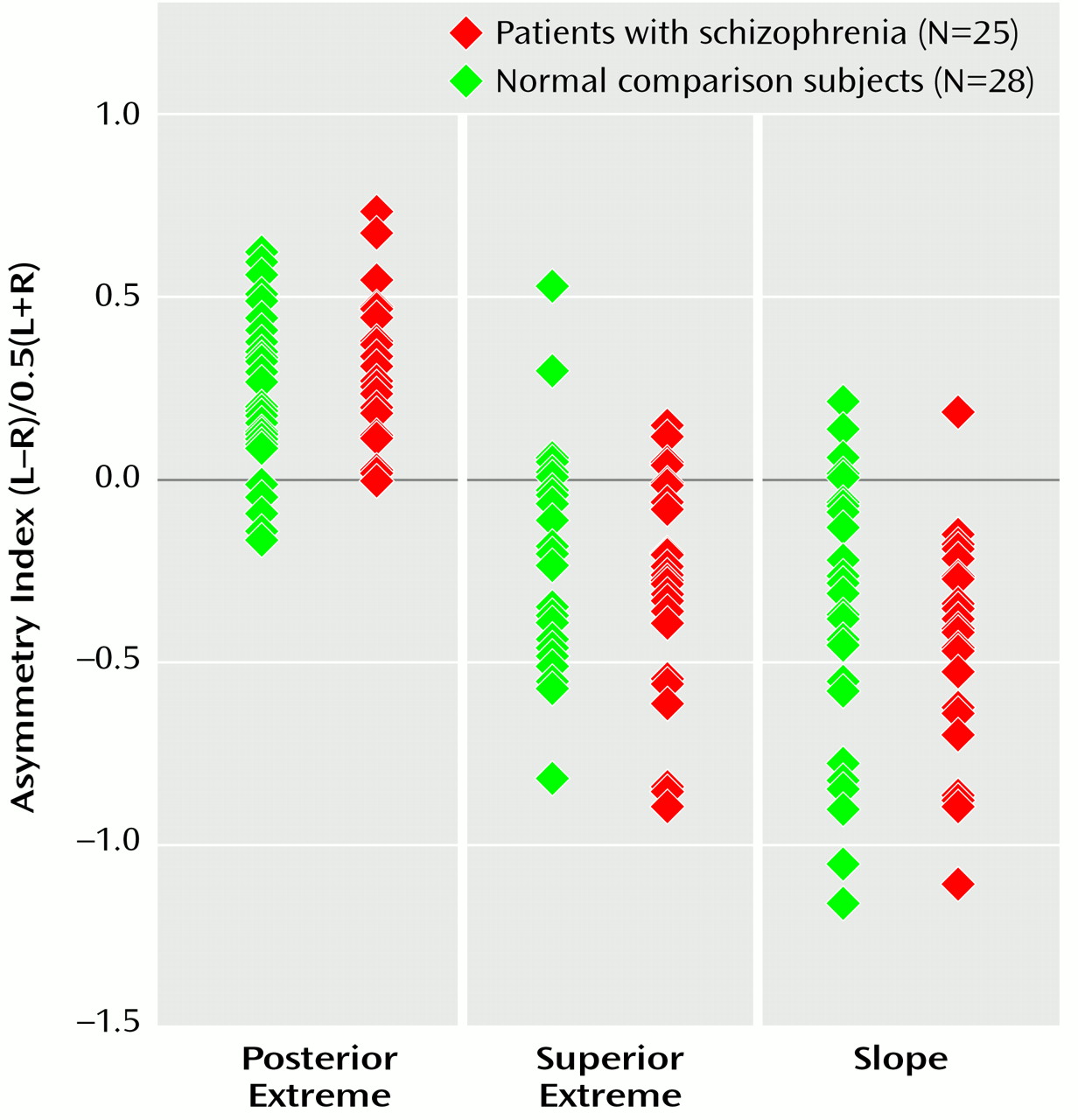
Post hoc power analyses revealed that empirically measured effect sizes for asymmetries were extremely large: f=0.90, f=0.60, and f=0.80 for the sylvian fissure posterior extent, superior extreme, and slope, respectively, with power between 0.90 and 0.99. Hemisphere-by-diagnosis interactions for these measures, however, showed extremely small empirically measured effect sizes (f<0.10). The probability of type II error for such small effects was therefore greater than 0.50. If these effect sizes are truly representative, our analyses indicate that only unreasonably large study groups would likely reveal effects showing small differences in sulcal asymmetries, even with alpha at 0.05. Notwithstanding, if the diagnosis-by-hemisphere effects were large, as implied by previous study results, our study would have had reasonable power to detect group differences in asymmetry.
These findings suggest that 1) sylvian fissure asymmetries at the cortex that include the planum parietale, which follows the terminal ascending ramus, do not reflect less than normal planum temporale asymmetry in schizophrenia
(54) and 2) group differences in perisylvian sulcal asymmetries, if present at the cortex, are likely so small that they are of minimal diagnostic importance (
Figure5 and
Figure 6). Supporting our findings, Bartley et al.
(58) used similar sylvian fissure measurements and found normal hemispheric asymmetries in monozygotic twins discordant for schizophrenia. Furthermore, DeLisi et al.
(3) reported somewhat less laterality in the horizontal component of the sylvian fissure in schizophrenia (although the difference was not statistically significant) but no difference for the vertical segment. Finally, we found the sylvian fissure superior extreme to be more inferior in the patients than in the comparison subjects but not to exhibit diagnosis-by-hemisphere effects.
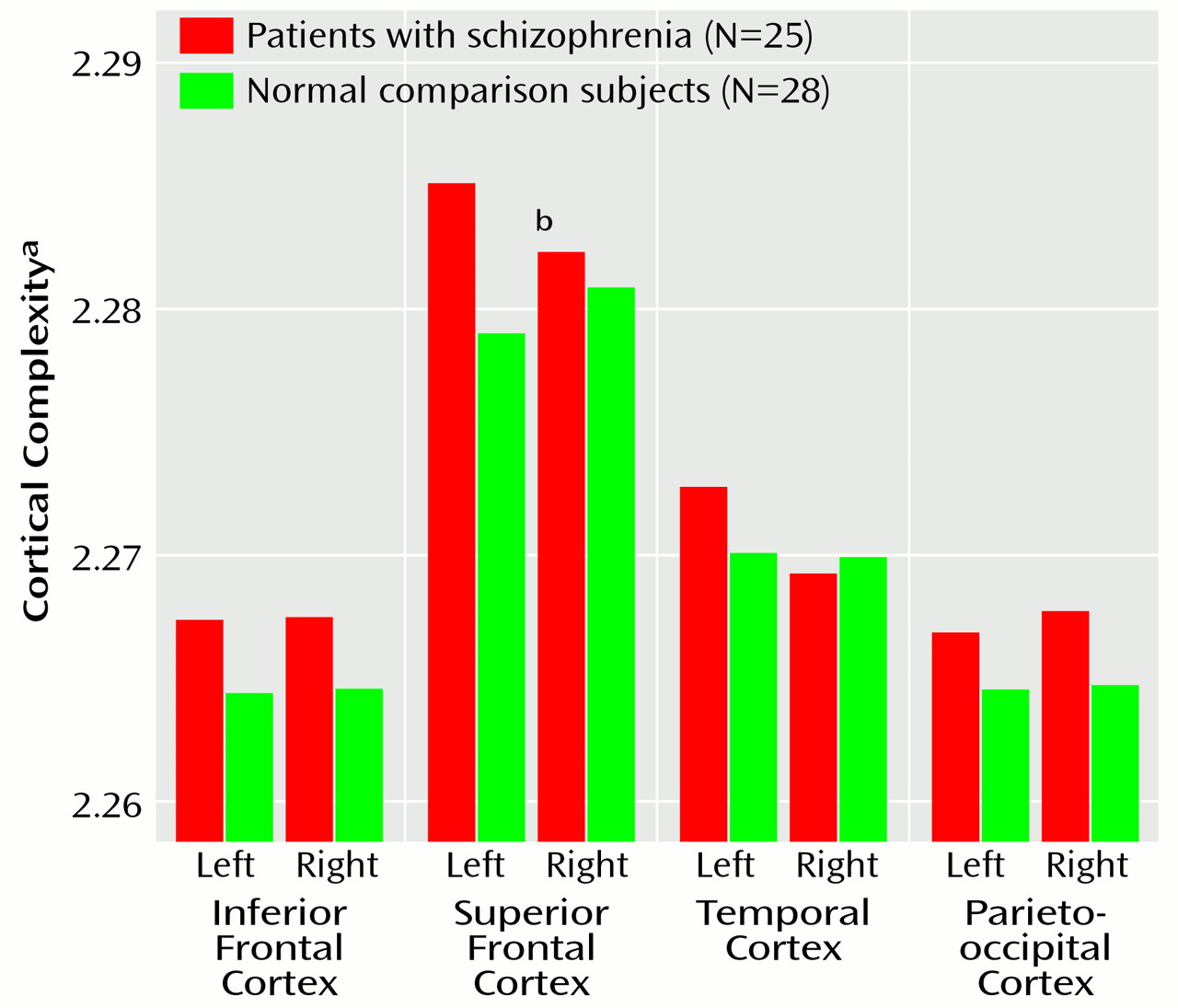
Cortical Complexity
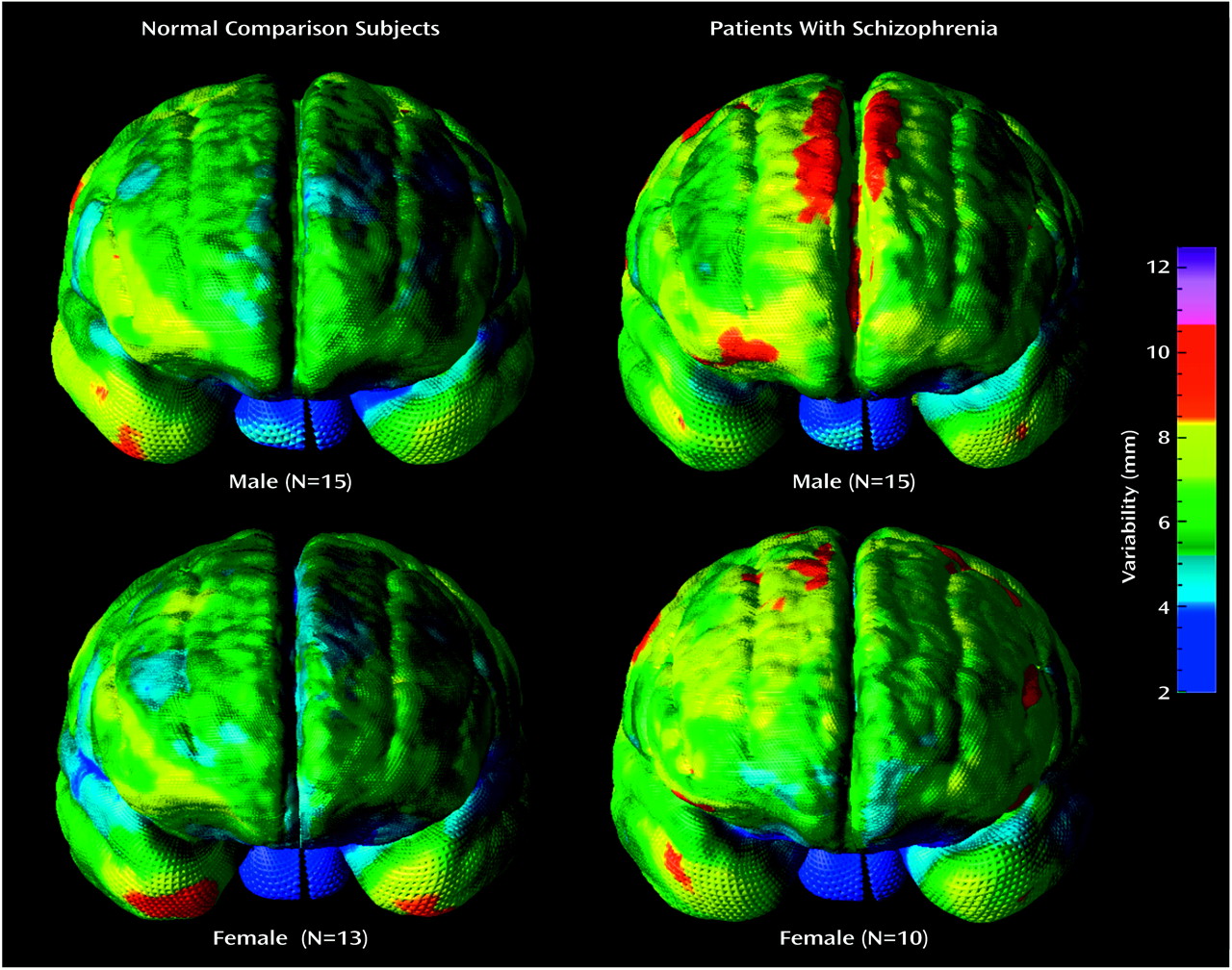
Cortical complexity reflects the number of primary, secondary, and tertiary sulcal bifurcations in a region and may reveal potentially different patterns of gyral geometry that become manifest during development or disease
(27,
40). Procedures for cortical surface averaging may underestimate interindividual differences in sulcal continuity
(38) that could discriminate diagnostic groups. Kikinis and colleagues
(61), for example, reported greater than normal vertical orientation and interruptions in the patterns of left hemisphere temporal sulci in schizophrenia. Furthermore, studies have shown hemispheric differences in frontal complexity in schizophrenia, which appear to interact with gender
(16,
17). For instance, significantly greater right hemisphere prefrontal complexity was found in male patients than in comparison subjects
(17) when a gyrification index
(62,
63) representing the ratio of the outer (superficial) to the inner (deep) perimeter of a cortical slice was determined. In contrast, we report abnormal asymmetry in superior frontal regions in patients (left hemisphere greater than right). Cortical complexity indices, however, showed average differences between patients and comparison subjects (except right hemisphere temporal lobe) (
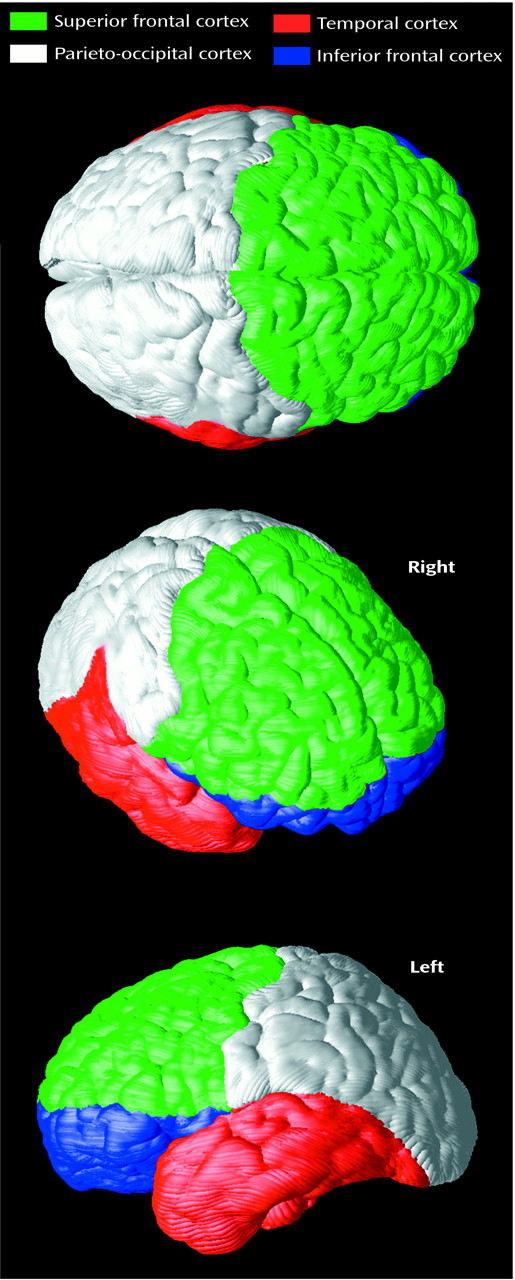
Figure 7) that were at least in the same direction as those indicating greater complexity in right frontal and left temporal regions in schizophrenia
(17,
61). Notwithstanding, different methods may make cross-study comparisons of complexity measures unsuitable. For example, here fractal complexity was measured in three dimensions such that group differences in gyral geometry in the vertical, horizontal, and lateral planes could be detected. That is, complexity was not computed on the basis of a single two-dimensional plane of reference defined by a particular, noncontiguous brain slice. Moreover, gyral complexity was measured across the cortical surface in anatomically homologous regions in each subject.
Greater cortical complexity in male than female subjects was found in inferior frontal regions. Other investigators
(62,
64) have failed to find differences in cortical complexity across gender in normal populations, but again, methodological differences between studies exist. For example, gyral complexity has been computed by using the a ratio of total cortical surface area to brain volume
(64), which may not be sensitive to the same features as the three-dimensional approach used here. Furthermore, in an earlier postmortem study
(62), only temporal regions were assessed in addition to the gyral complexity from coronal slices over the entire cortex. It is therefore possible that gender effects may have been overlooked in the inferior frontal cortices. Clearly, new studies may establish whether sexually dimorphic development processes, in specific cortical regions, influence gyral complexity.