Clozapine, the prototypical atypical antipsychotic, is rarely associated with elevation in prolactin level or extrapyramidal side effects
(1). The mechanism by which clozapine avoids these side effects is not clear. According to one explanation, clozapine does not give rise to prolactin elevation or extrapyramidal side effects because it blocks only a low level of dopamine D
2 receptors, and this level of D
2 receptor occupancy is not sufficient to give rise to these side effects (the low D
2 hypothesis)
(2–
4). This hypothesis would predict that if the D
2 receptor occupancy of patients taking clozapine were increased, prolactin elevation and extrapyramidal side effects might ensue. In contrast, another explanation for the lack of extrapyramidal side effects and prolactin elevation with clozapine is that the drug’s effects on serotonin 5-HT
2 or other receptors overcome the side effects related to D
2 receptor occupancy (the D
2 attenuation hypothesis)
(5). According to the second hypothesis, even if the D
2 receptor occupancy in patients taking clozapine were in the range usually associated with prolactin elevation and extrapyramidal side effects, the compensatory effects of clozapine on other receptors would prevent the expression of these side effects.
The difference between these two explanations is not only of academic interest but is of considerable practical importance. Nearly a third of the patients who take clozapine receive another antipsychotic, despite very limited scientific evidence about the efficacy and hazards of these combinations
(6). To address these issues, we sought answers to two questions: 1) does the addition of a typical antipsychotic increase the D
2 receptor occupancy of patients receiving clozapine? and 2) do patients receiving clozapine experience the D
2-related side effects (prolactin elevation and extrapyramidal side effects) conventionally associated with high D
2 receptor occupancy?
Method
The study was approved by the Human Subject Use Review Committee of the University of Toronto, and subjects participated after providing written consent by means of approved forms and procedures. The subjects in the study reported here were involved in an ongoing larger study of strategies for clozapine augmentation. The subjects were patients with DSM-IV schizophrenia who had been treated previously with antipsychotics, but, due to inadequate clinical response, were switched to treatment with clozapine. Included in the study were five male patients, age 25–39 years, who met the following criteria: 1) continuous treatment with clozapine for more than 3 months, 2) a clozapine plasma level >500 nM, and 3) no concomitant antipsychotic treatment.
At baseline, patients’ dopamine D
2 receptor occupancy was evaluated by means of [
11C]raclopride and positron emission tomography (PET). The scans were done 12–14 hours after patients took their regular dose of clozapine. Patients’ baseline clozapine plasma level and prolactin level were measured, and their level of extrapyramidal side effects was rated with the Simpson-Angus scale
(7) and the Barnes akathisia scale
(8). Four mg of haloperidol, as a nightly dose, was added to their regimen, and the patients were followed for a total of 6–8 weeks while receiving the combined treatment. In the fourth week, the patients had another PET scan to assess dopamine D
2 receptor occupancy, their clozapine and haloperidol plasma levels and prolactin level were measured, and the ratings of extrapyramidal side effects were repeated. At 6–8 weeks, all measures, except PET scanning, were repeated.
The PET scans to estimate dopamine D
2 receptor occupancy were obtained after the injection of 10 mCi of high-specific-activity [
11C]raclopride (300–1600 Ci/mmol) through the use of a bolus-plus-infusion protocol. The methods employed were the same as those described in previous studies examining the D
2 receptor occupancy of haloperidol, risperidone, olanzapine, and clozapine
(3,
4). Clozapine and haloperidol plasma levels were measured by using a liquid/liquid extraction followed by a liquid chromatography–mass spectrometry analysis (St. Joseph’s Health Centre, London, Canada). Prolactin level was determined by using a commercially available two-site chemoluminometric immunoassay (Ciba-Corning Diagnostics, Medfield, Mass.).
Results
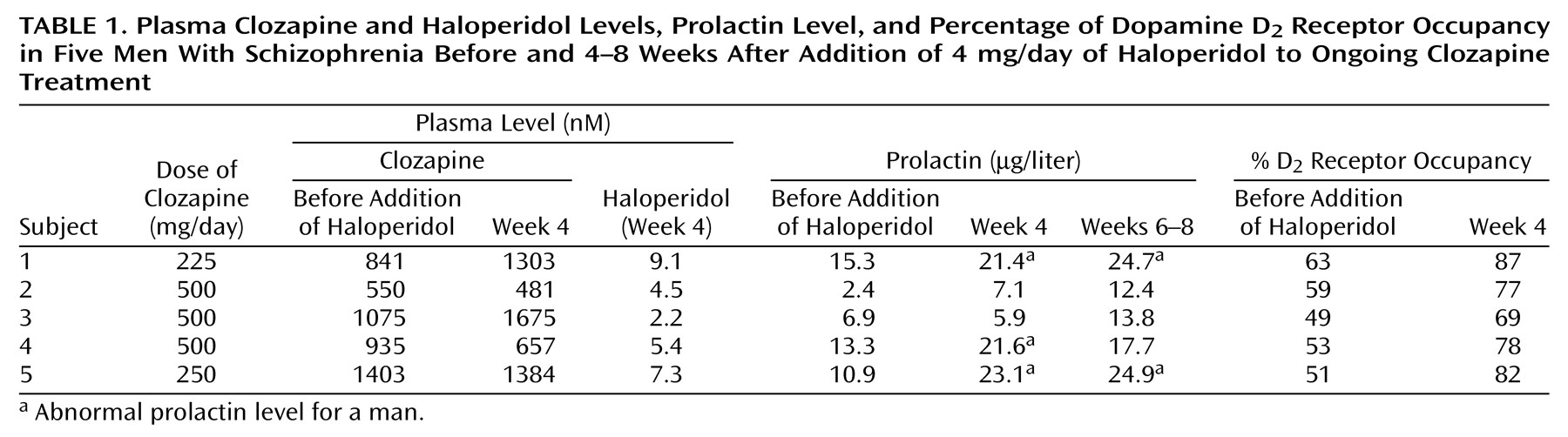
All five subjects completed the study. The results are reported in
Table 1. The addition of 4 mg/day of haloperidol resulted in a mean haloperidol plasma level of 5.7 nM (SD=2.6) and no significant change in mean clozapine plasma level (baseline mean=966 nM, SD=313; week 4 mean=1100 nM, SD=507; week 6 mean=926 nM, SD=162) (F=0.44, df=2, 8, p=0.65). Each subject showed an increase in the percentage of D
2 receptor occupancy, and the group mean increased from 55% (SD=6%) at baseline to 79% (SD=6%) at week 4 (paired t test t=9.97, df=4, p=0.001). The level of D
2 receptor occupancy at week 4 was not related to the plasma level of clozapine (R
2=0.02, F=0.07, df=1, 3, p=0.81) but was well predicted by the plasma level of haloperidol (R
2=0.98, F=122.68, df=1, 3, p=0.002).
The change in D2 receptor occupancy was associated with an increase in the mean prolactin level from 9.7 μg/liter (SD=5.1) at baseline to 15.8 μg/liter (SD=8.5) at week 4 and 18.7 μg/liter (SD=5.8) at week 6 (repeated measures test for a within-subject change in prolactin level: F=11.13, df=2, 8, p=0.007). At baseline, the patients receiving clozapine had prolactin levels in the normal range for men (2.1–17.7 μg/liter). However, after the addition of haloperidol, three patients had clinically abnormal levels.
The subjects’ mean global scores on the Barnes akathisia scale were 0.2 (SD=0.4) at baseline, 0.2 (SD=0.4) at week 4, and 1.0 (SD=1.4) at week 6. Their mean total scores on the Simpson-Angus scale were 0.8 (SD=0.8) at baseline, 1.6 (SD=1.5) at week 4, and 1.0 (SD=1) at week 6. The changes were not statistically significant. At a clinical level, patient 1 (with 87% D2 receptor occupancy at week 4) developed akathisia that necessitated treatment, and patient 2 (with 77% D2 receptor occupancy at week 4) showed mild cogwheeling that did not require any intervention.
Discussion
The addition of haloperidol to ongoing treatment with clozapine significantly elevated D
2 receptor occupancy, and this change was associated with significant prolactin elevation. Although several uncontrolled studies and case reports (reviewed in reference 6) have described effects of combining antipsychotics with clozapine, we could find only one previous study that examined this issue systematically
(9). This study reported an increase in prolactin level when sulpiride, a specific D
2 blocker, was added to clozapine. Our data support this earlier observation and add to it by showing that the increase in prolactin is associated with an increase in dopamine D
2 receptor occupancy.
The results with respect to extrapyramidal side effects are not conclusive and, given the open nature of the ratings, should be regarded with caution. At baseline, the average level of D
2 receptor occupancy in the subjects receiving clozapine was 55%, and no patient’s level of D
2 receptor occupancy exceeded 63%. When such low levels are obtained with haloperidol
(4) or with raclopride
(10) (both relatively specific D
2 blockers), no extrapyramidal side effects are observed, even though the drugs are typical antipsychotics. Thus, the low level of D
2 receptor occupancy in clozapine monotherapy is a sufficient explanation for the lack of extrapyramidal side effects associated with clozapine. This does not rule out the possibility, demonstrated in animal studies
(11) and suggested in data for humans
(12), that clozapine may have additional antiparkinsonian properties (through anticholinergic or other features), but these properties are not needed to explain clozapine’s lack of parkinsonian features. Although the patients in the study may not have shown significant extrapyramidal side effects while taking both clozapine and haloperidol, they clearly exhibited high D
2 receptor occupancy. It is possible that such a combination may give rise to dopamine supersensitivity or longer-term movement disorder effects that are not seen with clozapine alone
(13,
14). As this issue has not yet been empirically resolved, the outcomes in our study should be interpreted with caution.
Two limitations of this study should be highlighted. First, although the effect of combined treatment on prolactin level was significant even within this small study group, the data on extrapyramidal side effects were not as conclusive. A study involving a larger sample of patients, a higher dose of haloperidol (that would result in levels of D
2 receptor occupancy in the range of 85%–90%, clearly above the threshold for extrapyramidal side effects), and blinded assessment of extrapyramidal side effects would provide a better test of this hypothesis. Second, current PET technology does not permit the direct assessment of drug occupancy at the anterior pituitary dopamine D
2 receptors, which are responsible for the increase in prolactin level. We used the level of striatal D
2 receptor occupancy as a surrogate. This is a reasonable substitute, given that no significant differences have been found in the in vitro and in vivo (animal data) affinity of the receptors in the two regions
(15) and that previous studies have reported a robust empirical association between striatal receptor occupancy and prolactin elevation
(4,
16). However, pituitary receptors lie outside the blood-brain barrier, and, therefore, a direct assessment of pituitary receptor occupancy would provide a better understanding of the relationship between antipsychotic-induced D
2 receptor occupancy and prolactin elevation.
In summary, our data show that when clozapine is combined with modest doses of haloperidol, D2 receptor occupancy is similar to that observed with haloperidol alone. This level of D2 receptor occupancy is associated with robust prolactin elevation, suggesting that clozapine’s freedom from prolactin elevation is mainly a function of its low D2 receptor occupancy and is not due to its effects on other receptor systems. Further, patients receiving this combination of medications showed levels of D2 receptor occupancy associated with typical antipsychotics, a finding that raises the possibility that such a combination may lead to a higher incidence of tardive dyskinesia than would be observed in patients receiving clozapine alone. Since such combinations are rather commonplace in clinical practice, controlled studies that examine the efficacy and safety of such combinations are urgently required.