Several studies have reported that the concentration of the dopamine metabolite homovanillic acid in cerebrospinal fluid is lower in depressed patients than in healthy comparison subjects and that this difference is particularly pronounced in patients with marked psychomotor retardation (e.g., reference
1). These findings suggest that central dopamine function might be impaired in these patients. Furthermore, antidepressant drugs that enhance dopamine transmission might improve depression in patients with psychomotor retardation
(2). Although such preliminary observations could have treatment implications, no study that we are aware of has directly investigated presynaptic dopamine function in patients with different clinical subtypes of depression. We assessed presynaptic dopamine function by using positron emission tomography (PET) and 6-[
18F]fluorodopa ([
18F]DOPA) in depressed patients with psychomotor retardation, in depressed patients with high impulsivity, and in healthy comparison subjects.
Method
Twelve right-handed inpatients with a major depressive disorder diagnosed according to DSM-IV criteria and with a depression score >25 on the Montgomery-Åsberg Depression Rating Scale
(3) were recruited from consecutive admissions at three teaching hospitals. Only depressed patients with either marked depressive retardation (score >18 on the Depressive Retardation Rating Scale
[4]) or marked impulsivity (scores >10 on the loss-of-control subscale of the Depressive Mood Scale
[5]) and >10 on the Tyrer anxiety scale
[6]) were included. Thus, two groups with clearcut depression subtypes were defined: 1) six depressed patients with psychomotor retardation, who had high affective flattening and low loss-of-control scores on the Depressive Mood Scale subscales (two male patients and four female patients; mean age=51.8 years, SD=7.6; mean Montgomery-Åsberg Depression Rating Scale score=30.5, SD=3.9; mean Depressive Retardation Rating Scale score=22.0, SD=3.2; mean Depressive Mood Scale affective flattening subscale score=15.5, SD=5.4; mean Depressive Mood Scale loss-of-control subscale score=6.0, SD=3.6; and mean Tyrer anxiety scale score=13.7, SD=4.6) and 2) six depressed patients with high impulsivity and low retardation scores (one male patient and five female patients; mean age=40.2 years, SD=8.7; mean Montgomery-Åsberg Depression Rating Scale score=27.7, SD=3.1; mean Depressive Retardation Rating Scale score=11.7, SD=4.2; mean Depressive Mood Scale affective flattening subscale score=7.7, SD=7.4; mean Depressive Mood Scale loss-of-control subscale score=14.5, SD=3.3; and mean Tyrer anxiety scale score=20.3, SD=3.1). Although both groups had comparable mean scores on the Montgomery-Åsberg Depression Rating Scale (Mann-Whitney U test, U=9, p=0.15), clinical differences were significant on all other scales: Depressive Retardation Rating Scale (U=1, p=0.006), Depressive Mood Scale affective flattening subscale (U=5, p=0.04), Depressive Mood Scale loss-of-control subscale (U=36, p=0.004), and Tyrer anxiety scale (U=3, p=0.02).
Only drug-free patients (N=6, three in each group) and patients taking selective serotonin reuptake inhibitors (SSRIs) (N=6, three in each group) were included. Patients who had been treated in the previous month with drugs known to directly modulate dopamine neurotransmission were excluded. Other exclusion criteria were neurological and general medical conditions, pregnancy, and DSM-IV axis I comorbidity.
Ten right-handed healthy comparison subjects (four male subjects and six female subjects; mean age=42.2 years, SD=12.1) were selected from a group of community volunteers. The comparison group was similar to the patient group in age (Kruskal-Wallis test, H=3.5, df=2, p=0.17) and gender (χ2=0.95, df=1, p=0.62). The comparison subjects had no history of neurological or psychiatric conditions.
After complete description of the study to the subjects, written informed consent was obtained.
Anatomic magnetic resonance imaging (MRI) and [
18F]DOPA PET brain scans were performed
(7). Briefly, in the MRI protocol, 124 T
1-weighted images (1.5-mm thickness) were acquired with a 1.5-T Signa scanner (General Electric, Milwaukee). After a transmission scan for attenuation correction and injection of 2–5.6 mCi of [
18F]DOPA (prepared as previously described
[8]; mean=3.4 mCi, SD=0.9, in comparison subjects; mean=3.6 mCi, SD=1.2, in depressed patients with impulsivity; and mean=3.7 mCi, SD=0.8, in depressed patients with psychomotor retardation, Kruskal-Wallis test, H=0.27, df=2, p=0.87), 63 slices (2.5-mm thick) were obtained with an ECAT-HR+ tomograph (Siemens, Knoxville, Tenn.). Data for nine time frames of 10 minutes each were collected. Attenuation- and decay-corrected PET images were summed and aligned with the MRI images. Investigators who were blind to the clinical ratings determined regions of interest on the four or five aligned MRI slices in which the caudate and putamen were readily visible, and the regions were copied onto the PET images. Occipital cortex regions of interest, in which [
18F]DOPA uptake was assumed to be negligible, were used as reference regions. Regional [
18F]DOPA uptake K
i values were computed from the regional time-activity curves obtained between 30 and 90 minutes by using the Patlak graphical analysis method
(9).
Group comparisons for quantitative variables were performed by using the Kruskal-Wallis nonparametric test. Pairwise comparisons were performed with the Mann-Whitney U test. Significance was set at 0.05, two-tailed.
Results
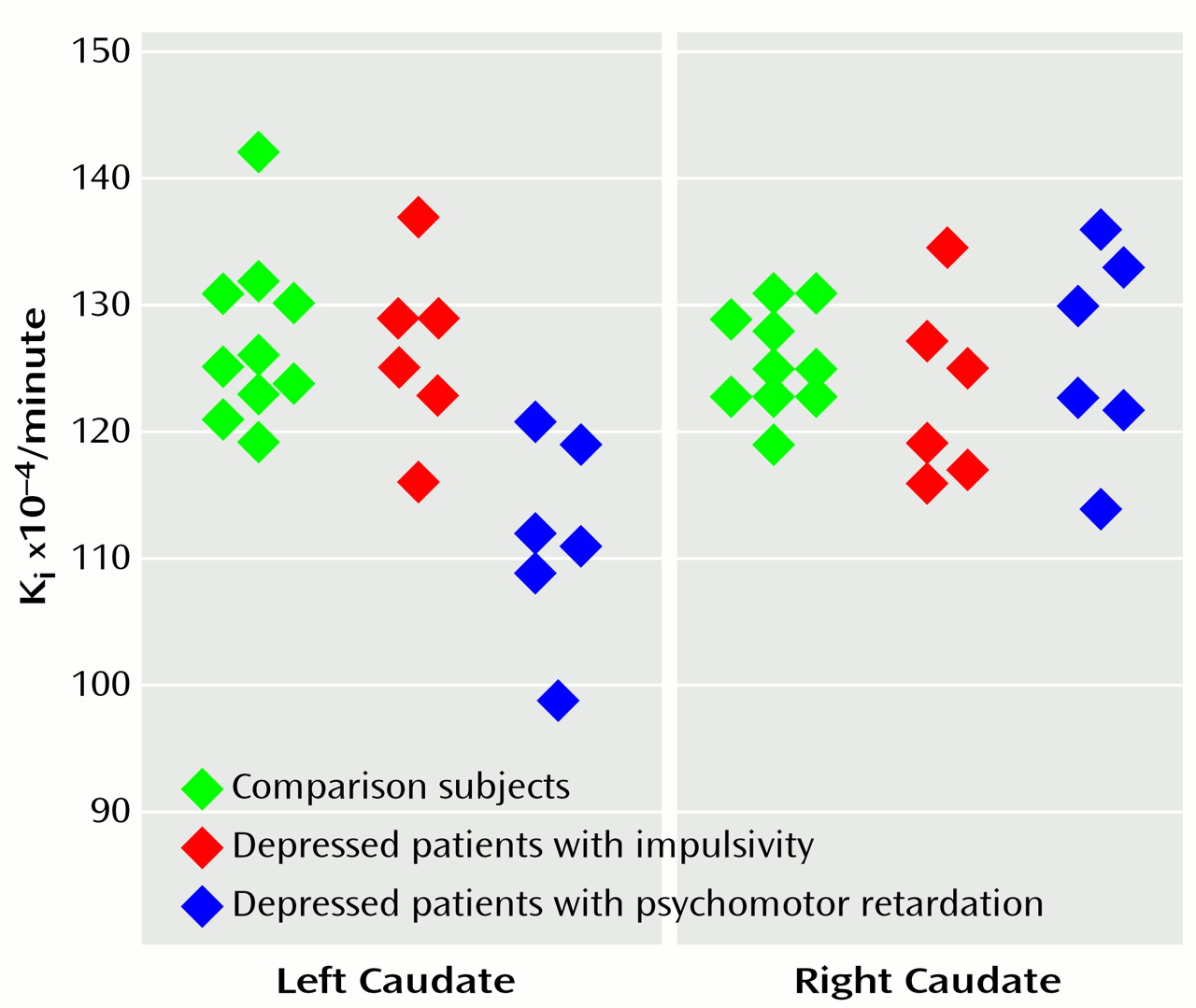
The mean uptake Ki values in the patients with psychomotor retardation, the patients with impulsivity, and the comparison subjects were, respectively, 0.0126 (SD=0.0008), 0.0123 (SD=0.0007), and 0.0126 (SD=0.0004) in the right caudate (Kruskal-Wallis test, H=0.85, df=2, p=0.65); 0.0112 (SD=0.0008), 0.0127 (SD=0.0007), and 0.0127 (SD=0.0007) in the left caudate (H=10.5, df=2, p=0.005); 0.0129 (SD=0.0010), 0.0123 (SD=0.0007), and 0.0130 (SD=0.0009) in the right putamen (H=2.1, df=2, p=0.34); and 0.0124 (SD=0.0009), 0.0123 (SD=0.0004), and 0.0126 (SD=0.0008) in the left putamen (H=1.4, df=2, p=0.49).
For the left caudate, comparisons showed that the mean K
i value in the patients with psychomotor retardation was significantly lower than that in the impulsive patients (U=2, p=0.01) and in the comparison subjects (U=2, p=0.002). The mean K
i values in the left caudate of the impulsive patients and the comparison subjects were not significantly different (U=28, p=0.83) (
Figure 1).
There was no effect of age on Ki values in either the caudate (r=0.17, N=22, z=–0.77, p=0.26) or the putamen (r=0.27, N=22, z=1.21, p=0.62).
Discussion
Decreased presynaptic dopamine function was found in the left caudate of depressed patients with affective flattening and psychomotor retardation. The caudate is part of a loop involving basal ganglia, anterior cingulate, thalamus, and prefrontal cortex that has been hypothesized to modulate mood
(10), and lesions of the left caudate have been associated with a higher frequency and severity of depression
(11). Our results are consistent with findings of a negative correlation between depressive symptoms and left striatal [
18F]DOPA uptake in patients with schizophrenia
(12), decreased caudate glucose metabolism in depressed patients
(13), and a negative correlation between psychomotor retardation and regional cerebral blood flow in the left prefrontal cortex in depressed patients
(14).
Although previous treatment with SSRIs in some patients might have had an indirect effect on dopamine transmission, it is unlikely that such an effect would involve the left caudate only and appear only in depressives with psychomotor retardation.
Thus, although the number of patients in this study was small, the results suggest a link between dopamine hypofunction in left caudate pathways and depressive psychomotor retardation with affective flattening. Further studies are needed to examine the role of the caudate in depressive disorders and to determine whether antidepressants that directly enhance dopamine transmission would be useful in treating these patients.