Over the past six decades, epidemics of methamphetamine abuse have occurred worldwide
(1–
4). Chronic methamphetamine users show psychiatric signs, including a psychotic state and an anxiety-disorder-like state, under conditions of intoxication, withdrawal, or both. These psychiatric states are sometimes prolonged as residual symptoms, easily exacerbated by methamphetamine reuse or by psychological stress in some chronic users
(3,
5–
8). The prolongation of psychosis and susceptibility to relapse in chronic methamphetamine users have often been regarded as manifestations of preexistent schizophrenia
(9), although differences in symptoms and morbid risk between subjects with schizophrenia and methamphetamine users with psychosis have also been demonstrated
(3).
There are three major dopaminergic systems, the nigrostriatal, mesolimbic, and mesocortical systems, which are related to coordinated movements and mentation
(10,
11). Given acutely, methamphetamine increases dopamine levels in the synaptic cleft, mainly by inhibiting action of the dopamine transporter
(12,
13). Postmortem study has shown that chronic methamphetamine use results in reduction of dopamine transporter density in the caudate/putamen and nucleus accumbens
(14). Positron emission tomography (PET) study has also shown the reduction of dopamine transporter density in the caudate/putamen of chronic methamphetamine users
(15), although a history of polydrug use might exert some influence on the PET results.
In this study, we selected subjects who abused only methamphetamine (i.e., monodrug users) and investigated the dopamine transporter density of the brain during a period of abstinence from methamphetamine. We used PET and 2-β-carbomethoxy-3β-(4-[
11C] fluorophenyl)tropane ([
11C]WIN-35,428), which is a cocaine analogue with high specificity to dopamine transporter
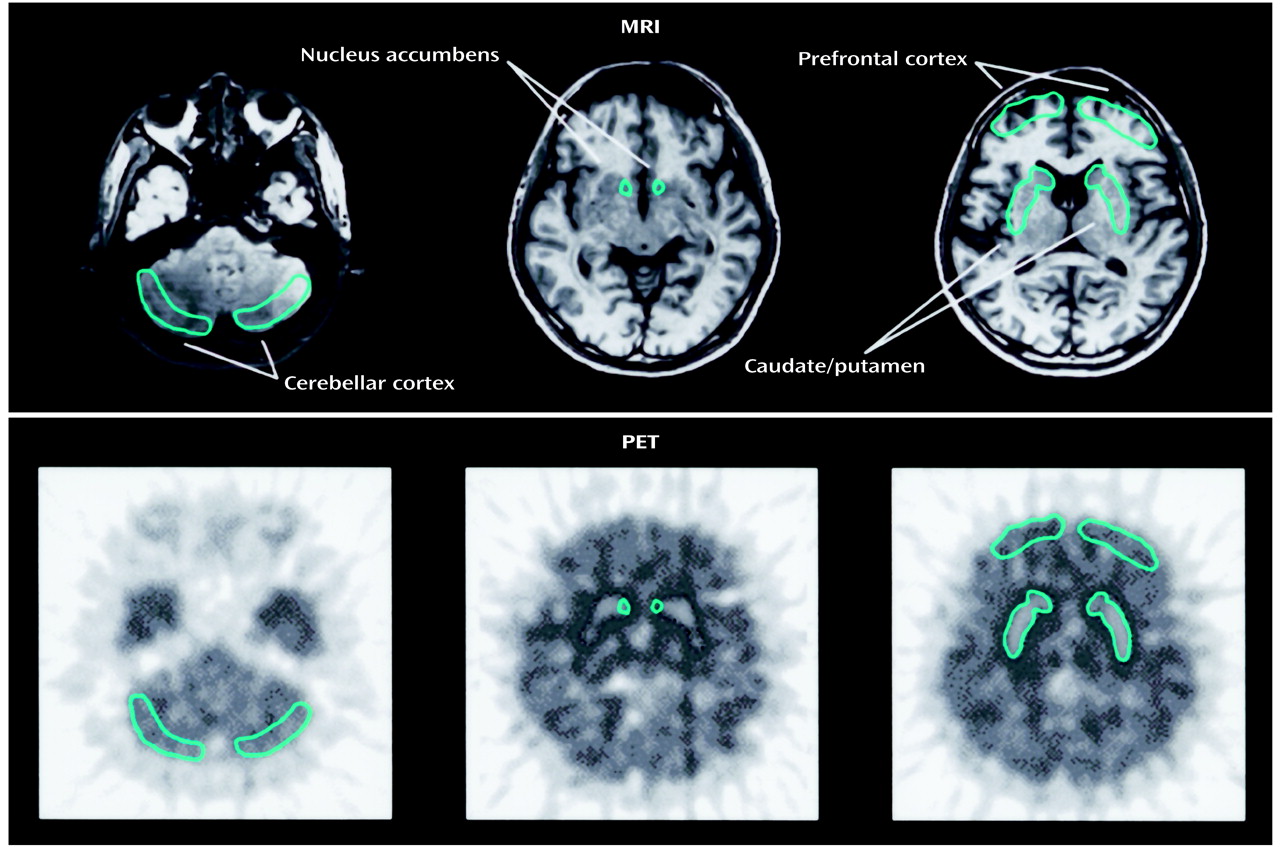
(16). In addition to the caudate/putamen and nucleus accumbens, we investigated the prefrontal cortex, which receives terminals of the mesocortical dopaminergic system. Further, we examined the relationship between the dopamine transporter density and clinical characteristics, including psychotic symptoms and craving for methamphetamine, in methamphetamine users.
Results
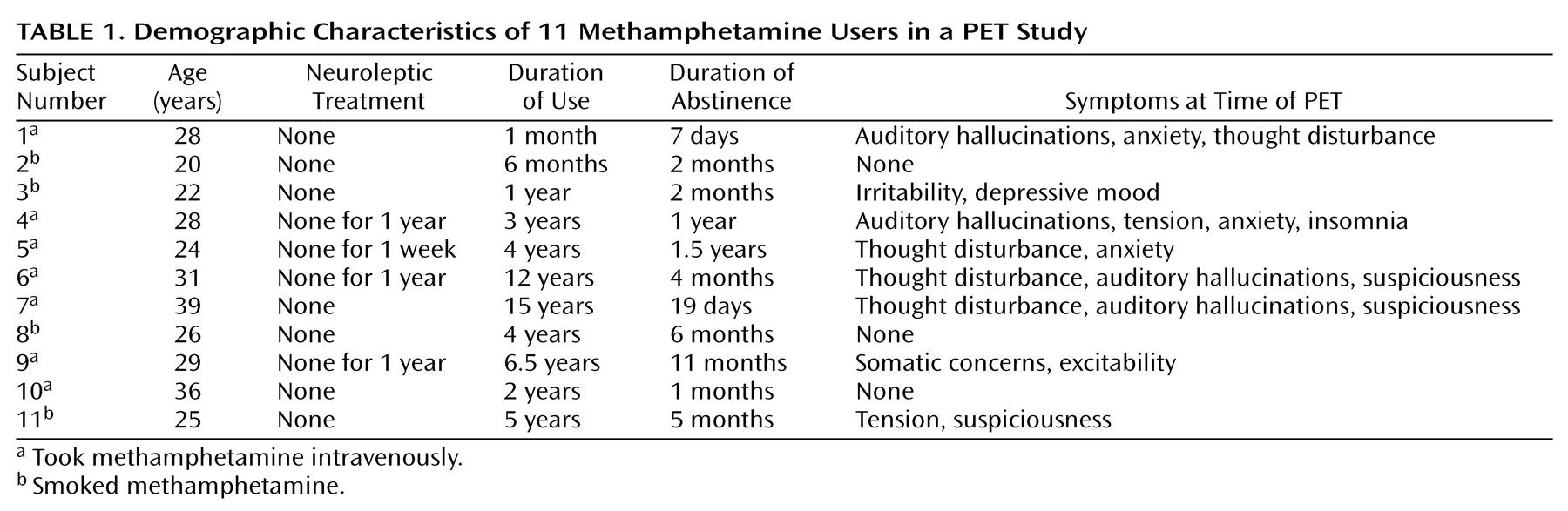
The demographic characteristics of methamphetamine users are shown in
Table 1. No significant difference in age or education levels was observed between methamphetamine users and comparison subjects. None of the methamphetamine users had a history of psychotic symptoms before the use of methamphetamine, and none showed negative symptoms during the present examination. Eight methamphetamine users (numbers 1, 3–7, 10, and 11) had previously experienced methamphetamine psychosis. Over the 2 weeks preceding the PET examination, three methamphetamine users (numbers 2, 8, and 10) had no psychiatric symptoms; four (numbers 3, 5, 9, and 11) had persistent anxiety-disorder-like, but not psychotic, symptoms; and three (numbers 4, 6, and 7) had persistent psychotic symptoms such as auditory hallucinations and delusions. One methamphetamine user (number 1) began to show psychotic symptoms during the use of methamphetamine and still exhibited the symptoms on the day of the PET examination. None of the methamphetamine users was taking neuroleptics at the time of PET examination, although four (numbers 4–6 and 9) had received neuroleptic treatment previously.
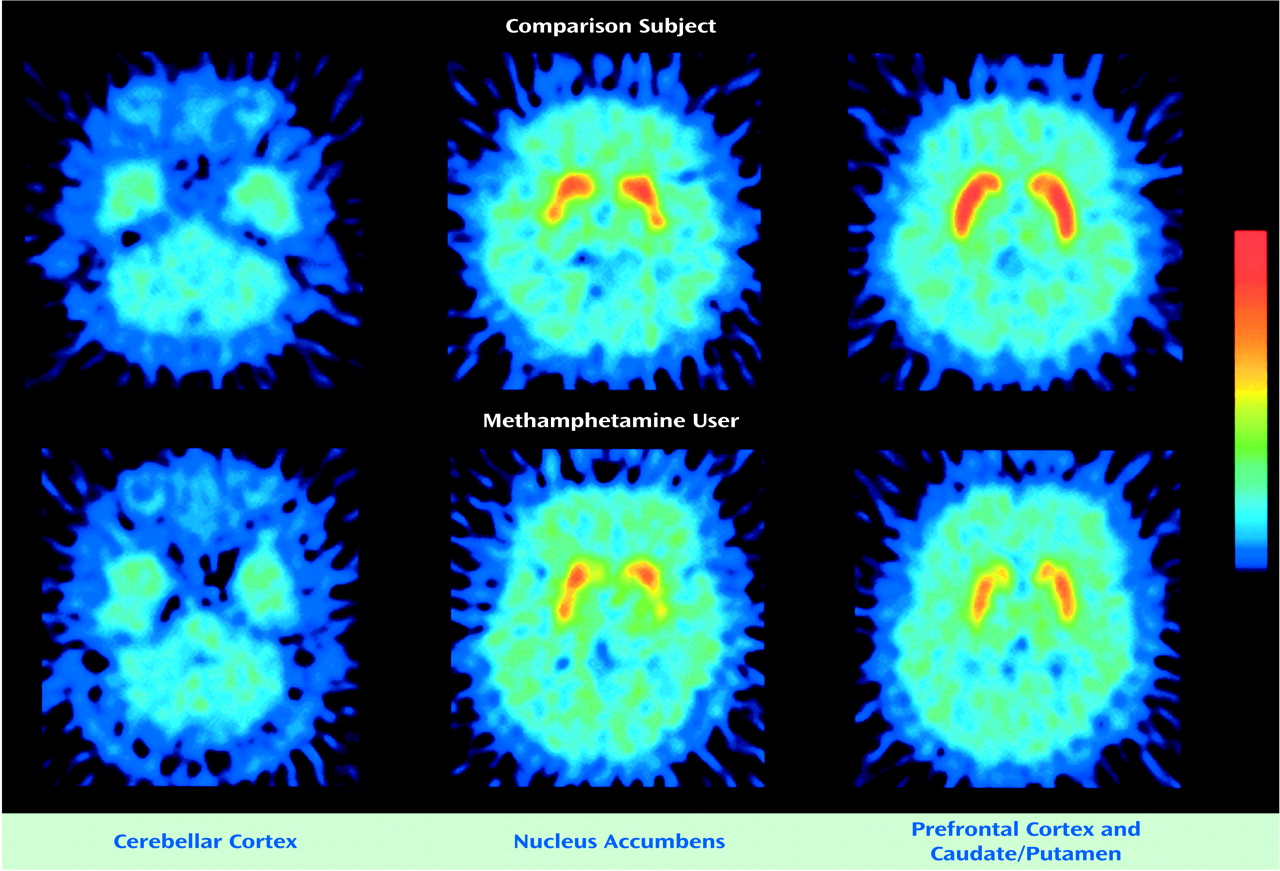
Figure 2 shows PET images from a comparison subject and from the methamphetamine user with the lowest dopamine transporter binding potential (number 7). Both images show high accumulation of radioactivity in the caudate/putamen and nucleus accumbens and low accumulation in the prefrontal cortex and cerebellar cortex. The radioactivity in the caudate/putamen and nucleus accumbens was lower in the methamphetamine user than in the comparison subject.
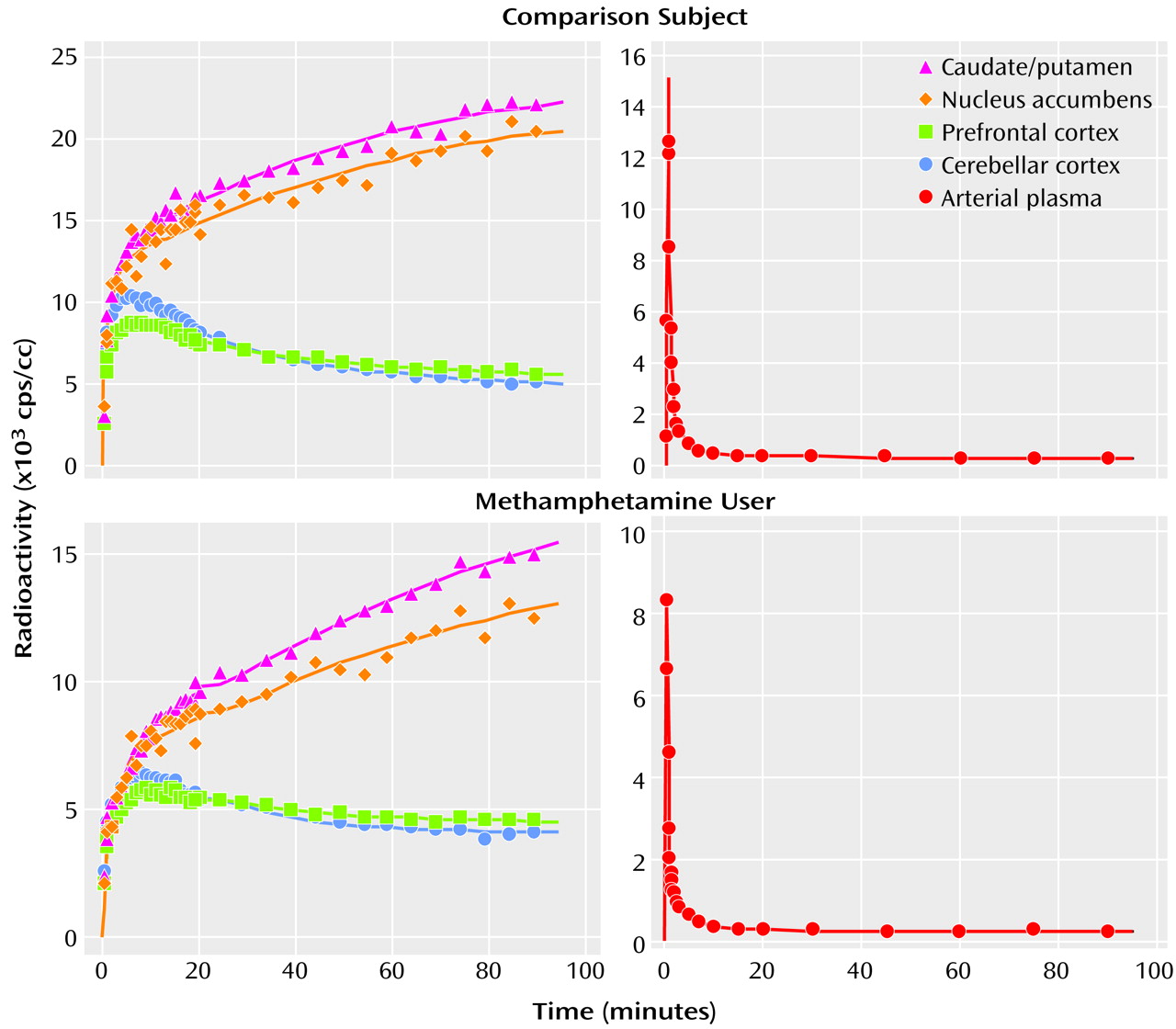
Figure 3 shows representative curves of radioactivity in the brain and in the arterial plasma from a comparison subject and a methamphetamine user. Following a venous injection of the tracer, the radioactivity in the caudate/putamen and nucleus accumbens increased until the end of measurement, whereas that in the prefrontal cortex and cerebellar cortex decreased gradually after reaching a peak within 10 minutes.
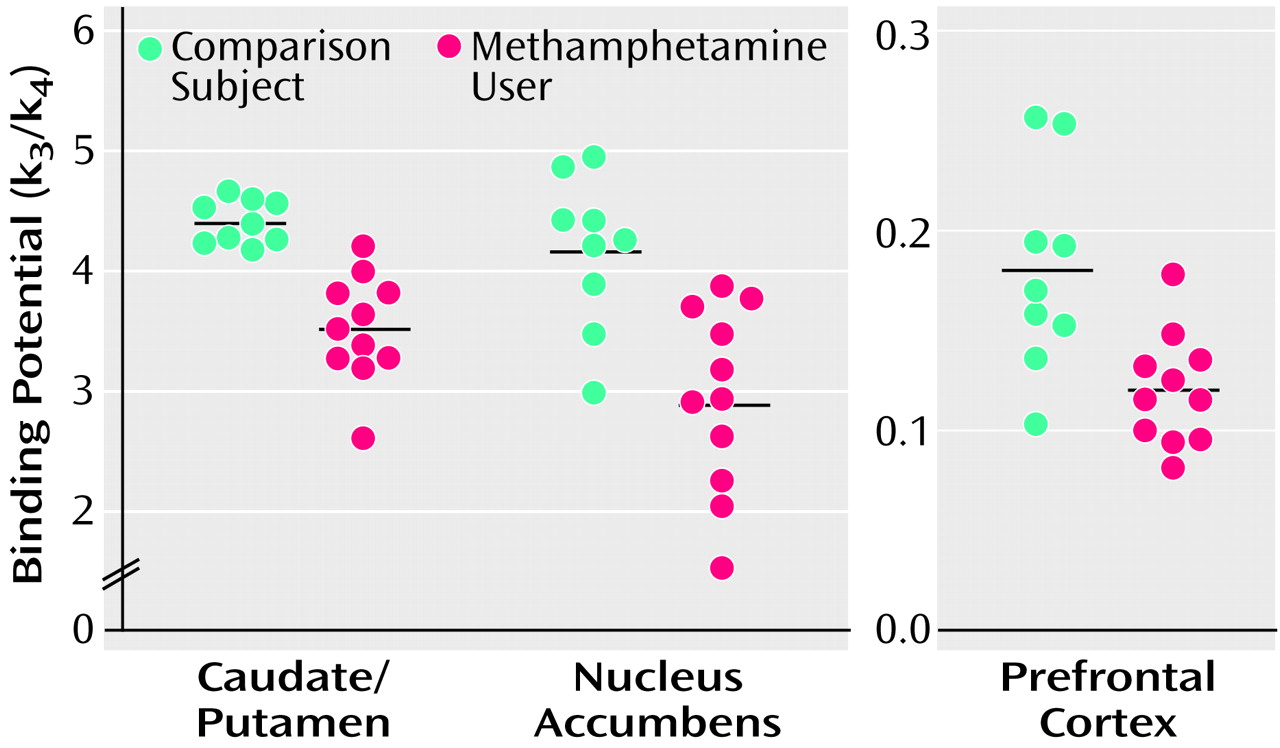
Values of dopamine transporter binding potential in the caudate/putamen, nucleus accumbens, and prefrontal cortex are shown in
Figure 4. The mean binding potential for the caudate/putamen was 4.40 (SD=0.18) in comparison subjects and 3.51 (SD=0.45) in methamphetamine users. The latter value constitutes a 20.2% difference. In the nucleus accumbens, the mean binding potentials in comparison subjects and methamphetamine users were 4.16 (SD=0.64) and 2.93 (SD=0.77), respectively. The latter value represents a 29.6% difference. The mean binding potential for the prefrontal cortex was 0.18 (SD=0.05) in comparison subjects and 0.12 (SD=0.03) in methamphetamine users. The value of the latter was 33.3% lower. The dopamine transporter binding potentials for the caudate/putamen, nucleus accumbens, and prefrontal cortex were significantly lower in methamphetamine users than the comparison subjects (caudate/putamen: U=1.0, N=20, p<0.001; nucleus accumbens: U=8.5, N=20, p<0.001; prefrontal cortex: U=23.5, N=20, p<0.05, Mann-Whitney U test).
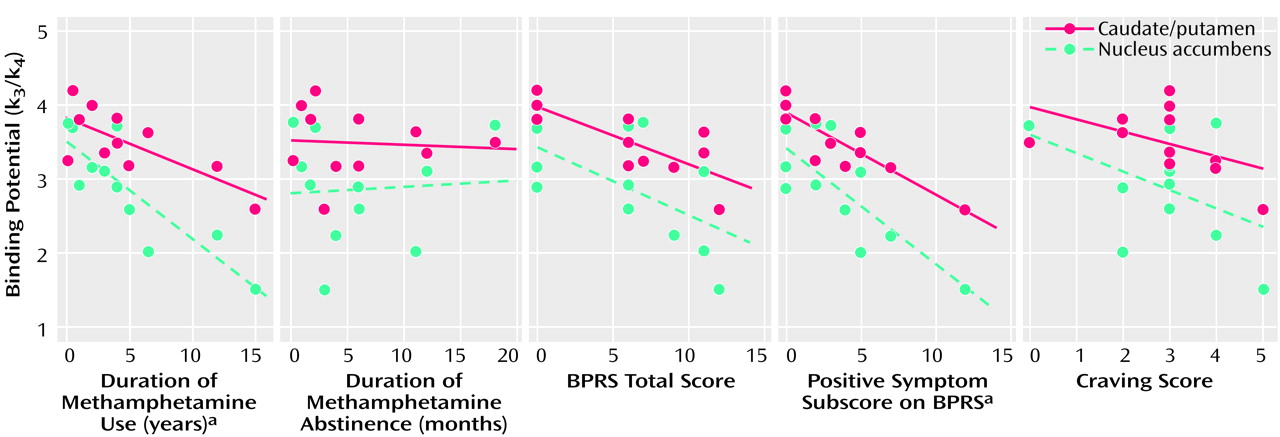
There was a significant correlation between the clinical data for the methamphetamine users and the dopamine transporter binding potential in the caudate/putamen and in the nucleus accumbens (
Figure 5), but no correlation was found between the clinical measures and prefrontal cortex dopamine transporter binding potential (data not shown). The dopamine transporter binding potential in the caudate/putamen and nucleus accumbens had a significant negative correlation with the duration of methamphetamine use (caudate/putamen: Kendall’s tau=–0.48, df=10, p<0.05; nucleus accumbens: Kendall’s tau=–0.73, df=10, p<0.01). No significant correlation was observed between the duration of abstinence and the dopamine transporter binding potential for either the caudate/putamen or the nucleus accumbens. The caudate/putamen dopamine transporter binding potential, but not the nucleus accumbens binding potential, showed a significant negative correlation with the total BPRS score (Kendall’s tau=–0.62, df=10, p<0.05). There was a significant negative correlation between the score on the BPRS subscale for positive symptoms and the dopamine transporter binding potential for the caudate/putamen and the nucleus accumbens (caudate/putamen: Kendall’s tau=–0.73, df=10, p<0.01; nucleus accumbens: Kendall’s tau=–0.50, df=10, p<0.05). The subjective craving score was not significantly correlated with the dopamine transporter binding potential for either the caudate/putamen or the nucleus accumbens.
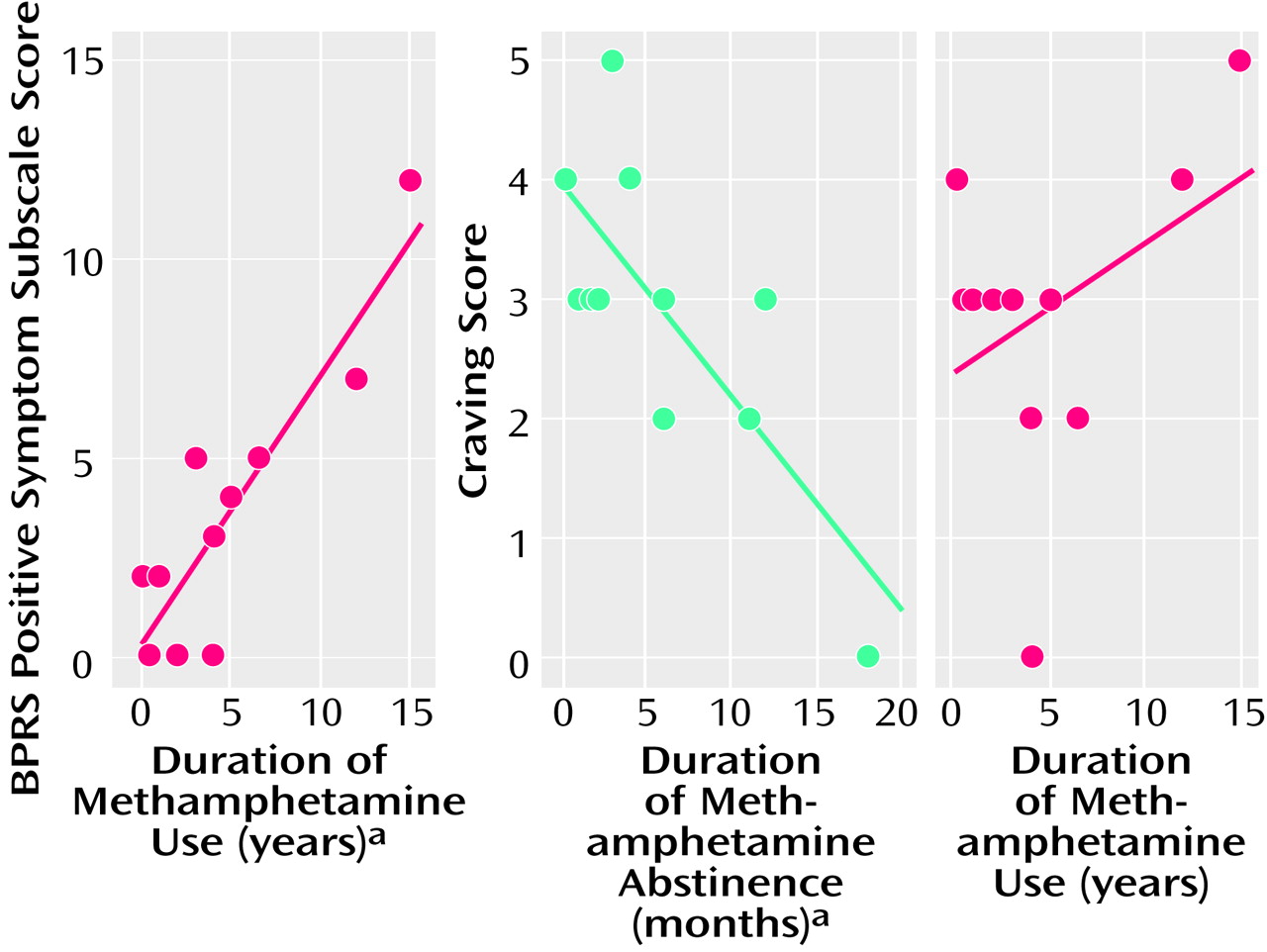
There was a significant correlation between the score on the BPRS subscale for positive symptoms and the duration of methamphetamine use (Kendall’s tau=0.64, df=10, p<0.01) (
Figure 6). The subjective craving score showed a significant negative correlation with the duration of abstinence from methamphetamine (Kendall’s tau=–0.50, df=10, p<0.05) but not with the duration of methamphetamine use (
Figure 6).
Discussion
In the present PET study, the rank order of the dopamine transporter density represented by binding potential observed in methamphetamine users and comparison subjects who did not use methamphetamine was caudate/putamen, then nucleus accumbens, then prefrontal cortex. The finding of low dopamine transporter density in the prefrontal cortex was compatible with the results of a previous PET study demonstrating very low density of dopamine D
2 receptors in the human prefrontal cortex
(24). The present results also show that in the caudate/putamen, nucleus accumbens, and prefrontal cortex, the dopamine transporter density of methamphetamine users was significantly lower than that of comparison subjects. Therefore, we suggest that in the human brain, methamphetamine causes reduction of the dopamine transporter density in the mesolimbic, mesocortical, and nigrostriatal dopaminergic systems, although the number of subjects was small in this study.
Our finding that dopamine transporter density was lower in the caudate/putamen and nucleus accumbens of the methamphetamine users may be in agreement with the results of previous postmortem and PET studies
(14,
15). With respect to the postmortem study
(14), however, it is unclear whether polydrug users were included as subjects. In addition, the cause of death was not clear in the postmortem study: in the case of overdose, several conditions would have to be considered in order to understand the results. In the PET study
(15), users of multiple drugs, including cocaine, cannabis, and LSD, were included as subjects, although these substances are known to cause psychosis and to influence neural transmission in the brain
(25).
In order to evaluate the specific effects of methamphetamine on the dopamine transporter density associated with psychiatric symptoms, we selected methamphetamine users who had abused only methamphetamine (i.e., not polydrug users). Some of the methamphetamine users had a history of treatment with neuroleptics for a psychiatric state, but previous studies have reported that neuroleptics have no effect on dopamine transporter density or affinity
(26,
27). In addition, the methamphetamine users in this study took the substance intravenously or by smoking. This difference in the method of methamphetamine intake might contribute to the correlation between the dopamine transporter change and the clinical measures, although methamphetamine possesses similar pharmacological characteristics whether administered intravenously or by inhalation
(28,
29). A larger study group and a more homogenous population would be needed to completely eliminate these factors.
In contrast to the dopamine transporter density for the caudate/putamen and nucleus accumbens, which was significantly correlated with some clinical characteristics, the prefrontal cortex dopamine transporter density did not correlate with any of the clinical markers, although it is generally accepted that the dopaminergic neurons in the prefrontal cortex are actively involved in the genesis and expression of a psychotic state
(10,
11). In addition, studies suggest the importance of cerebellar function for psychotic disorders
(30) and the presence of dopaminergic axons in certain lobules of the cerebellar vermis in primates
(31). However, in the present study, which used a ligand of [
11C]WIN 35,428, the dopamine transporter density in the cerebellar vermis was not quantitatively measured. To clarify the role of dopamine transporter in the prefrontal cortex and the cerebellar vermis for methamphetamine-related psychiatric symptoms, a larger study will be needed, along with the development of a new tracer with a higher specific-to-nonspecific binding ratio that allows low binding site density to be detected.
The dopamine transporter density for both the caudate/putamen and the nucleus accumbens decreased with increasing duration of methamphetamine use, suggesting that, in the habitual methamphetamine users in this study, the reduction of dopamine transporter density was dependent on the duration of methamphetamine use. Although the methamphetamine-induced change in dopamine transporter density appears to be influenced by the pattern and rate of methamphetamine use
(32), our findings did not help to clarify this issue. However, the methamphetamine users in this study used methamphetamine recreationally and had no history of toxic or high-dose methamphetamine use. Since methamphetamine-induced dopamine transporter reduction is known to be dose-dependent
(32,
33), the duration of methamphetamine use, as a determining factor of the reduction in dopamine transporter density observed in habitual methamphetamine users, may represent this dose dependency.
The severity of positive symptoms increased with the duration of methamphetamine use. Detoxification from methamphetamine in all users was confirmed by regular urine drug screening tests, including a test on the day of the PET examination, to establish that the psychiatric symptoms evaluated in this study were residual rather than acute symptoms induced by methamphetamine use. The present observations suggest that longer use of methamphetamine induces greater reduction in dopamine transporter density, which in turn is associated with a higher occurrence of residual psychiatric symptoms.
The dopamine transporter density of the caudate/putamen decreased with increasing total score and positive subscale score on the BPRS. The dopamine transporter density of the nucleus accumbens had no apparent correlation with the BPRS total score but decreased with increasing scores on the positive subscale. This finding suggests that the reduction in dopamine transporter density of both the caudate/putamen and nucleus accumbens may actively participate in the pathogenesis of residual psychiatric symptoms in methamphetamine users.
We found no significant correlation between the duration of abstinence, which lasted in our subjects from 1 week to 1.5 years, and dopamine transporter density in either the caudate/putamen or the nucleus accumbens. The previous PET study
(15) showed a significant reduction in the caudate/putamen dopamine transporter density in chronic methamphetamine users, some of whom had been abstinent for more than 3 years. These findings suggest that lasting reduction of brain dopamine transporter density could occur after habitual methamphetamine use.
In terms of craving for methamphetamine, a significant correlation was found with the duration of methamphetamine abstinence. However, no correlation was observed between the craving for methamphetamine and the duration of methamphetamine use. In addition, craving was not associated with dopamine transporter density in the nucleus accumbens, although it has been hypothesized that this structure is related to drug reward
(34). These findings are not inconsistent with our contention that craving for methamphetamine may be associated with reversible changes in the brain, such as changes in postsynaptic dopamine receptors, which are restored over time
(35,
36).
In a previous study using the same PET scanner and tracer as used here, the striatal dopamine transporter density in unmedicated patients with Parkinson’s disease showed a pronounced reduction of about 80%
(23). Methamphetamine users in this study, who had no symptoms of parkinsonism or other neurological abnormalities, showed a small reduction of caudate/putamen dopamine transporter of roughly 20%. Since striatal dopamine transporter declines with age
(37,
38), chronic methamphetamine users may have a greater risk for developing Parkinson’s disease as they age. Although small, the reduction in caudate/putamen dopamine transporter in the methamphetamine users in our current study was clearly associated with the development of psychiatric symptoms. In the case of methamphetamine users, as distinct from patients with Parkinson’s disease, the small reduction in caudate/putamen dopamine transporter appears to have a predictive value for the development of psychiatric signs. However, in order to strengthen the specificity and sensitivity of the caudate/putamen dopamine transporter reduction as a predictor for psychiatric symptoms, it will be necessary to evaluate another brain area as a negative control after a new tracer more highly specific to the dopamine transporter becomes available.
Several reports have indicated the possible involvement of the caudate/putamen in cognitive and personality disorders
(39,
40). In particular, a previous PET study
(41) showed an association between low dopamine transporter density and detached personality. Whether chronic use of methamphetamine influences or enhances such a personality trait is not known, but research into this question may provide further insight into the possible associations between caudate/putamen dopamine transporter density and psychiatric symptoms.
Oxidative dopamine products such as the dopamine quinones, which are increasingly formed under the condition of high concentration of intracellular dopamine
(42), are known to modify the proteins at the sites of cysteinyl residues. Dopamine transporter, which contains several cysteinyl residues, is one of the proteins at risk for this modification
(43), and methamphetamine is known to increase intracellular dopamine level by inhibiting vesicular transporter and monoamine oxidase B activity
(44). Such modification may partly explain the reduction of dopamine transporter density observed here, since the dopamine nerve terminals of chronic methamphetamine users are exposed to excessive dopamine levels for long periods of time.
In summary, our results clearly show parallelism between reduction of dopamine transporter density in the brain, which is associated with the duration of methamphetamine use, and the symptoms of a chronic psychotic state in methamphetamine users. Therefore, chronic methamphetamine use may contribute to the production of prolonged psychosis in methamphetamine users. However, dopamine transporter is only one part of the dopaminergic system, and we examined only one aspect of the dynamics of dopamine transmission. Indeed, in a previous animal study
(32), methamphetamine was reported to produce long-term reductions in serotonin transporter density, in addition to the decrease in dopamine transporter density. Therefore, changes in serotonin neurons, which have been implicated in many psychiatric symptoms
(45–
47), might have occurred in the methamphetamine users in the current study and might thus have influenced our results, including those on clinical symptoms. We also cannot rule out the possibility of preexisting condition of dopamine transporter reduction in methamphetamine users. Clearly, further work will be needed to define the causal mechanism of methamphetamine-associated psychosis.