The thalamus is involved in the processing of sensory inputs and a variety of interactions among cortical, subcortical, and brainstem nuclei
(1). The mediodorsal nucleus of the thalamus is among the brain regions of major interest in schizophrenia. Magnetic resonance spectroscopic imaging (MRSI) data in this brain region have shown variable results
(2–
6). Whereas significant bilateral reductions in
N-acetylaspartate (NAA) concentration and the NAA-to-creatine ratio in the thalamus of patients with schizophrenia have been reported by Omori et al.
(2) and Deicken et al.
(3), respectively, Bertolino et al.
(4,
5) did not find significant differences in this region. In a single-voxel study, Heimberg et al.
(6) found a trend toward lower NAA levels in the left thalamus. In addition, Omori et al.
(2) found a significantly lower ratio of choline-containing compounds to creatine in schizophrenic patients compared to healthy subjects.
The purpose of this study was to investigate whether lower concentrations of NAA, creatine plus phosphocreatine, and choline-containing compounds would be seen in the thalamic mediodorsal region of patients with schizophrenia. This finding appears crucial in verifying diminished thalamic function in schizophrenia and may provide a dynamic and noninvasive method for quantifying thalamic function over time.
Method
Fifteen patients (10 men and five women) who satisfied DSM-III-R as well as ICD-10 criteria for schizophrenia and who had a stable diagnosis for at least 2 years participated in this MRSI study. The mean age of the patients was 33.0 years (SD=7.0, range=23–47). All patients were evaluated at the Central Institute of Mental Health, were clinically stable for at least 6 months, and had been on a stable medication regimen for at least 3 months. Five of the 15 patients received typical neuroleptics and had never taken an atypical medication, whereas eight patients had received atypical medication for at least the last 3 months. Mean illness duration was 131.3 months (SD=88.3, range=6–285). All patients underwent separate structural magnetic resonance imaging (MRI) scans. Qualitative evaluation of the structural images by a neuroradiologist blind to subject status revealed no major structural abnormalities.
MRSI data of 15 healthy, age-matched subjects (eight men and seven women; mean age=31.9 years, SD=6.7, range=22–48) were obtained for comparison. None of the patients or comparison subjects had a history of head injury, organic mental disorder, neurological disorder, or alcohol or substance abuse. There were no significant group differences between patients and comparison subjects for age. All comparison subjects were free of psychiatric illness. Written informed consent was obtained after the purpose of the study and the procedures were explained to all participants. The study was approved by the university ethics committee.
The MRSI data were acquired by using a 1.5-T Magnetom VISION system (Siemens, Erlangen, Germany) with a standard circularly polarized head coil. An MRSI sequence with point-resolved spectroscopy volume was used with the volume centered on the thalamus. Measurement parameters (field of view=210 × 210 mm, slice thickness=15 mm, TE=135 msec, TR=1.5 seconds) resulted in a measurement time of 11 minutes.
For postprocessing of the MRSI data, an automated spectral fitting program
(7–
9) was used. Before the spatial Fourier transformation, k-space apodization was applied, which resulted in an effective voxel size of approximately 4 cm
3 and zero filling to 32 × 32 k-space points. Zero filling from 512 to 1,024 time domain data points and Gaussian multiplication corresponding to 0.6-Hz line broadening were carried out before the time domain Fourier transformation. The signals of NAA, creatine plus phosphocreatine, and choline-containing compounds were curve fit, and voxels from left and right thalamic mediodorsal regions of the thalamus, including the mediodorsal nucleus, were manually selected. For all selected voxels, the metabolite line width was well below 10 Hz.
Absolute integral values for NAA, creatine plus phosphocreatine, and choline-containing compounds were corrected for differential head coil loading by multiplication with the transmitter reference voltage
(10,
11). This yielded a semiquantitative measure, and thus metabolite ratios could be avoided.
Repeated measure analysis based on a general linear model was used for data analysis (SPSS for Windows release 10.0 [Chicago]). The dependent variable was the concentration estimate for each metabolite (NAA, creatine plus phosphocreatine, or choline-containing compounds), group was the between-subject factor, and side (left versus right) was the within-subject repeated measure factor.
Results
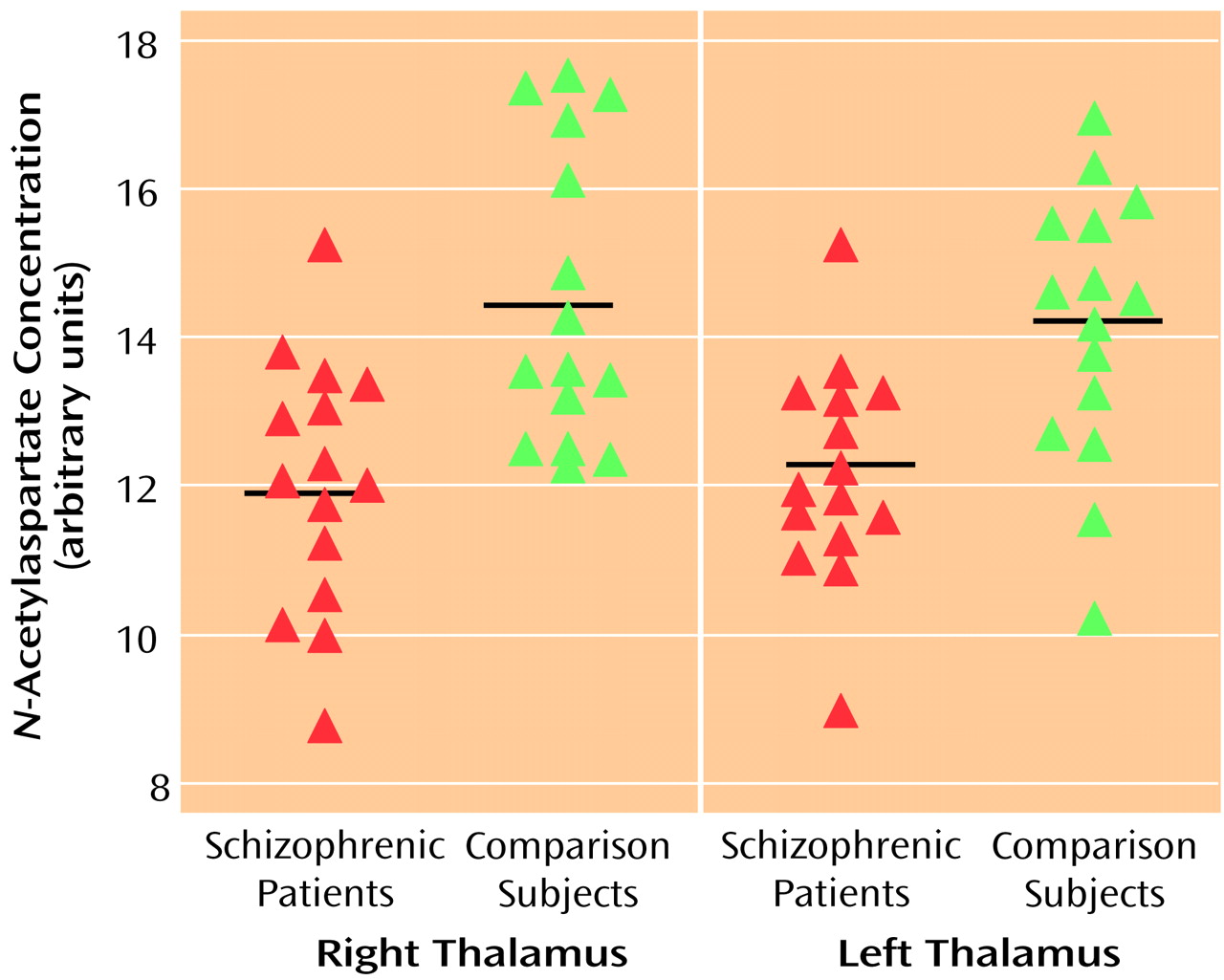
One voxel centered on each side of the mediodorsal region of the thalamus was selected for evaluation. As seen in
Figure 1, significantly lower concentrations of NAA were found in the schizophrenic patients relative to comparison subjects in both the right and the left thalamus (right thalamus: mean=12.0 [SD=1.7] versus 14.5 [SD=2.0], respectively; left thalamus: mean=12.2 [SD=1.5] versus 14.2 [SD=1.8], respectively).
Repeated measure analysis also revealed significant differences between the patients with schizophrenia and the comparison subjects in concentrations of choline-containing compounds (right thalamus: mean=7.9 [SD=0.81] versus 8.7 [SD=1.3], respectively; left thalamus: mean=7.2 [SD=1.2] versus 8.5 [SD=1.2], respectively) (F=8.6, df=1, 28, p=0.007).
Repeated measure analysis revealed no significant difference between the patients with schizophrenia and the comparison subjects in concentrations of creatine plus phosphocreatine (right thalamus: mean=6.4 [SD=0.8] versus 6.8 [SD=1.0], respectively; left thalamus: mean=6.3 [SD=1.1] versus 6.7 [SD=1.0], respectively) (F=2.6, df=1, 28, p<0.12).
No lateralized asymmetry of metabolites could be determined. No significant correlation between any of the metabolite signals and age or illness duration was found for patients and comparison subjects.
There was a significant positive correlation between left and right thalamic NAA concentrations in the patients with schizophrenia (r=0.53; F=5.2, df=1, 13, p<0.05) but not in comparison subjects (r=0.02; F=0.006, df=1, 13, p<0.94).
Discussion
MRI studies of thalamic volume have been inconsistent (see reference
1 and the references within). Hazlett et al.
(1) used MRI in combination with PET to show that while morphologic changes of the thalamus in the schizophrenic patients were only marginal, there were pronounced functional changes localized to subregions of the thalamus, most pronounced in the mediodorsal nucleus. Omori et al.
(2) and Deicken et al.
(3) reported lower NAA concentrations in the thalamus in schizophrenic patients. Deicken et al.
(3) pointed out that the discrepancy between their findings and previous MRSI studies
(4,
5) may be the result of differences in voxel selection. We are aware of the limitations provided by the still relatively coarse resolution of the MRSI data of 4 cm
3 and the lack of adjusting for heterogeneous tissue in the chosen voxel. Nevertheless, we have corroborated the finding of lower NAA concentrations in carefully selected voxels of the mediodorsal region of the thalamus in schizophrenic patients.
We also replicated the second finding reported by Deicken et al.
(3) of a significant correlation between right and left thalamic NAA measures in patients with schizophrenia. The findings of the present study further support the interpretation that neuronal dysfunction or loss in the mediodorsal nucleus in schizophrenic subjects affects both right and left sides to the same extent in an individual with schizophrenia.
These results are accompanied by the finding of significantly lower concentrations of choline-containing compounds in this region. Choline is thought to reflect the free phosphocholine and choline pool and as such is a putative marker of synthetic membrane formation, which in the CNS could be associated with synapse formation
(12). A decrease in choline suggests less synthetic activity or membrane formation and gives evidence for a lack of gliosis.
It is conceivable that these findings may be associated in part with chronic antipsychotic treatment. The evaluation of this influence is beyond the scope of the current study but should be investigated in future studies with a longitudinal design.