Among the clinical features of schizophrenia, disturbances in cognitive function may be the most persistent, disabling, and refractory to standard treatments
(1). In addition, cognitive deficits have been reported to serve as a premorbid marker for those at high risk for the development of schizophrenia
(2), and among the ill, the degree of cognitive impairment appears to be the best predictor of long-term outcome
(3). At least some of the cognitive abnormalities in schizophrenia appear to reflect dysfunction of the dorsal prefrontal cortex
(4,
5), and this dysfunction appears to be associated with deficits in gray matter volume and synaptic connectivity in this region
(6–
8).
However, the specific presynaptic elements of prefrontal cortex circuitry that are altered in schizophrenia are unclear. Axonal projections from the mediodorsal thalamic nucleus, the principal source of thalamic inputs to the prefrontal cortex
(9), are a likely candidate. For example, structural imaging studies have found thalamic volume deficits in subjects with chronic schizophrenia and a lengthy treatment history as well as never-medicated schizophrenic subjects
(10,
11), and in postmortem studies both the volume and neuronal number of the mediodorsal thalamic nucleus have been reported to be lower
(12–
14). Thus, fewer neurons in the mediodorsal thalamic nucleus could contribute to less synaptic connectivity in the prefrontal cortex of schizophrenic subjects. However, it is not known whether the missing thalamic neurons are local circuit or cortically projecting neurons and, if the latter, whether their lower number is actually associated with fewer mediodorsal thalamic nucleus axon terminals in the prefrontal cortex.
In contrast to animal models, the integrity of axonal projections from a specific brain region cannot be directly assessed in the human brain. However, the calcium-binding protein parvalbumin is expressed in neurons of the principal relay nuclei of the primate thalamus
(15), which project principally to cortical layers deep 3 and 4
(9). Consistent with these observations, studies in monkeys and humans have demonstrated that a subpopulation of parvalbumin-containing axon terminals in the prefrontal cortex have the synaptic specialization, laminar distribution, synaptic targets, and size that are characteristic of thalamic projections
(16). For example, approximately 50% of the parvalbumin-immunoreactive axon terminals in the middle layers (deep 3 and 4) of monkey prefrontal cortex area 9 form Gray’s type I or asymmetric (presumably excitatory) synapses. In contrast, all of the parvalbumin-immunoreactive axon terminals in the superficial layers (2 and superficial 3), which receive few direct inputs from the mediodorsal thalamic nucleus
(9), form the type II or symmetric synapses characteristic of inhibitory local circuit neurons. Thus, these data indicate that all parvalbumin-immunoreactive axon terminals in the superficial layers of the primate prefrontal cortex arise from intrinsic γ-aminobutryic acid (GABA)-containing neurons, whereas half of those in the middle layers represent projections from the thalamus.
Consequently, in this study we assessed the density of parvalbumin-immunoreactive axon terminals in the superficial and middle layers of prefrontal cortex area 9 from 20 subjects with schizophrenia and 20 matched comparison subjects. We hypothesized that the density of these terminals in the middle cortical layers of the subjects with schizophrenia would be lower, consistent with fewer axonal projections from the mediodorsal thalamic nucleus. In order to determine the specificity of these observations, similar studies were conducted in subjects with major depressive disorder and in monkeys after 9–12 months of haloperidol treatment.
Method
Subject Characteristics
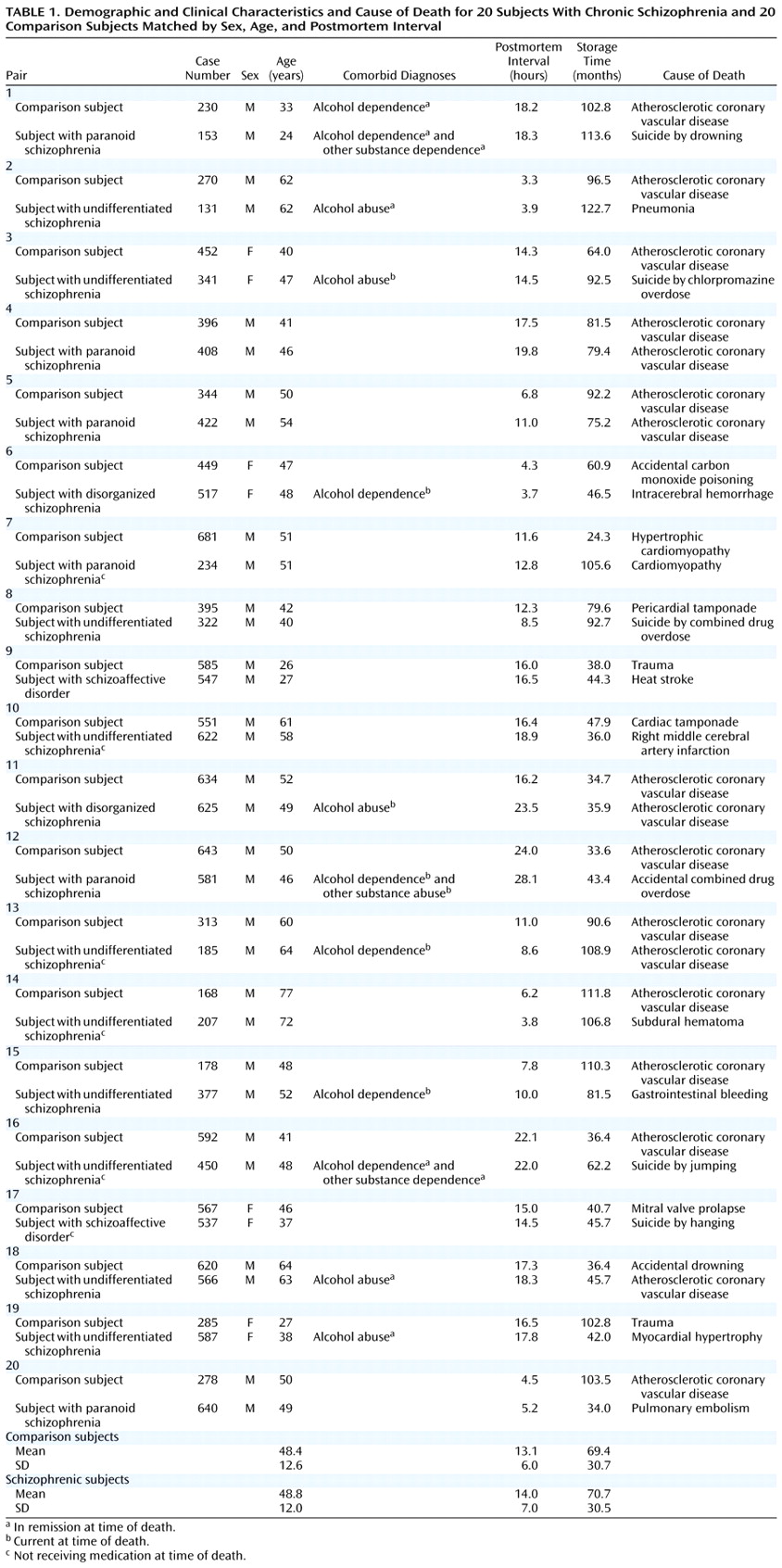
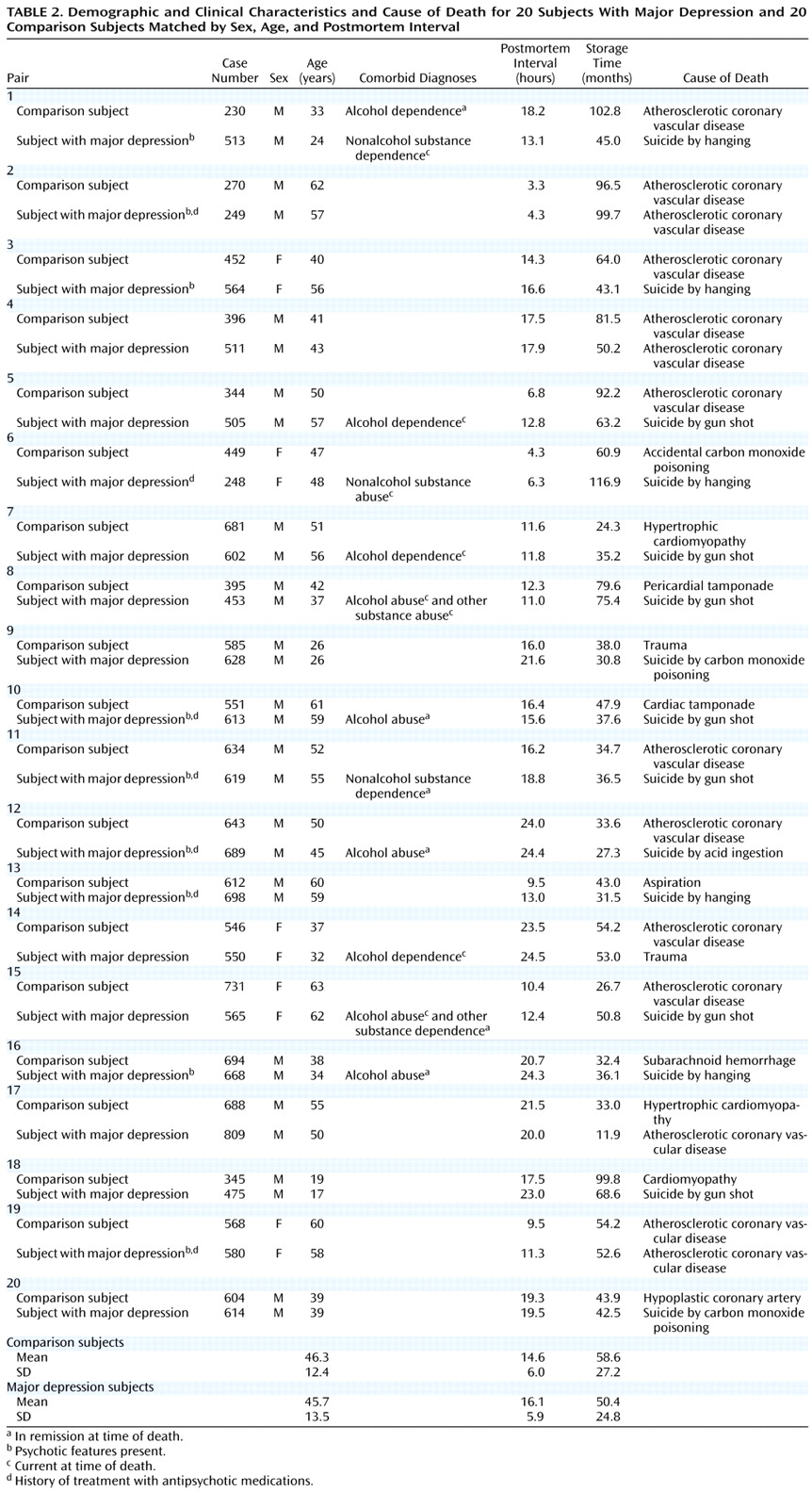
Brain specimens were obtained during autopsies conducted at the Allegheny County Coroner’s Office after obtaining consent from the surviving next-of-kin and following procedures approved by the University of Pittsburgh’s Institutional Review Board for Biomedical Research. Twenty pairs of schizophrenic and comparison subjects and 20 pairs of major depressive and comparison subjects—matched for sex, age, and postmortem interval—were used in two separate studies (
Table 1 and
Table 2). The pairs of schizophrenic and comparison subjects were completely matched for sex (16 male, four female), and the mean difference within pairs was 4.1 years (SD=3.2) for age and 1.9 hours (SD=1.8) for postmortem interval. The mean values of these subject groups (
Table 1) did not differ for either age (t=0.30, df=38, p=0.77) or postmortem interval (t=1.64, df=38, p=0.12). The pairs of major depressive and comparison subjects were also completely matched for sex (15 male, five female), and the mean differences within pairs was 4.0 years (SD=3.7) for age and 2.3 hours (SD=1.9) for postmortem interval. The mean values of these subject groups (
Table 2) did not differ in age (t=0.49, df=38, p=0.63), although the mean difference of 1.5 hours in postmortem interval was significant (t=2.5, df=38, p=0.02).
An independent panel of experienced clinicians arrived at consensus DSM-III-R diagnoses after reviewing medical records and the results of structured interviews conducted (after obtaining written informed consent) with family members of the deceased
(7). These interviews also revealed a history of alcohol dependence, in remission at the time of death, in one comparison subject (subject 230); no psychiatric disorders were present in the other comparison subjects. Eleven schizophrenic subjects and 11 major depressive subjects also had a history of an alcohol or other substance disorder; in six schizophrenic and seven major depressive subjects these diagnoses were current at the time of death (
Table 1 and
Table 2). Toxicology studies revealed plasma alcohol levels in two comparison subjects (0.01% and 0.03%), two schizophrenic subjects (0.12% and 0.13%), and five subjects with major depression (0.01%, 0.15%, 0.18%, 0.18%, and 0.35%); no other drugs of abuse were detected in any subject. Five subjects with schizophrenia (subjects 450, 622, 185, 537, and 207) had discontinued antipsychotic medications for 0.1, 0.1, 0.5, 0.8, and 10 years before death, respectively, and subject 234 had never taken psychotropic medications. Seven subjects with major depression had been treated with antipsychotic medications (
Table 2), but only subjects 689 and 698 were receiving them at the time of death. The mean age at the onset of illness was 27.2 years (SD=9.5) and 37.7 years (SD=12.8) for the subjects with schizophrenia and major depression, respectively, and the average duration of illness was 21.7 years (SD=10.4) and 8.3 years (SD=9.8), respectively. The brain specimens used in this study were obtained from a community-based population; consequently, the majority of subjects (15 schizophrenic and 20 comparison subjects, and 17 major depressive and 19 comparison subjects) died suddenly outside of a hospital setting.
Neuropathological examination revealed abnormalities in three subjects with schizophrenia (infarction in the distribution of the inferior branch of the right middle cerebral artery [subject 622]; left parietal subdural hematoma [subject 207]; and vascular malformation and intracerebral hemorrhage in the right temporal lobe and acute and subacute infarctions in the left thalamus [subject 517]), one normal comparison subject (bilateral contusions in parietal cortex [subject 585]), and five subjects with major depression (trauma to left basal ganglia and temporal lobe [subject 602]; trauma to left temporal lobe [subject 613]; trauma to bilateral temporal lobes [subject 565]; trauma to the medulla and cerebellum [subject 475]; and trauma to brainstem and bilateral temporal lobes [subject 619]). However, left prefrontal cortex area 9, the region under study, and the left mediodorsal thalamic nucleus appeared to be unaffected with the exception of a subacute mediodorsal thalamic nucleus infarction in subject 517. Additionally, thioflavin-S staining revealed a few senile plaques in subjects 207, 313, 564, 612, 620, and 731, but neither clinical nor neuropathological criteria for Alzheimer’s disease were met in any subject
(17).
Tissue Preparation and Processing
The left frontal lobe of each brain was cut into 1.0-cm thick coronal blocks. Blocks were immersed in cold 4% paraformaldehyde in 0.12 M phosphate buffer for 48 hours and then stored in a cryoprotectant at –30°C
(7). Mean tissue storage time in cryoprotectant did not differ between schizophrenic and comparison subjects (t=0.18, df=38, p=0.86) or between major depressive and comparison subjects (t=1.7, df=38, p=0.10) (
Table 1 and
Table 2). In addition, previous studies have demonstrated that tissue storage under these conditions does not alter immunoreactivity for the protein examined in this study
(18,
19). Blocks were sectioned coronally at 40 μm, and every 10th section was stained for Nissl substance with thionin. These sections were used to identify the location of area 9 on the superior frontal gyrus according to cytoarchitectonic criteria
(7,
20).
Floating tissue sections containing area 9 were processed for parvalbumin immunoreactivity by using the avidin-biotin procedure and the Vectastain ABC Elite kit (Vector Laboratories, Burlingame, Calif.) as previously described
(19,
21). Tissue sections from each matched pair of subjects were always processed together but with the investigator blind to diagnosis. For the 12 comparison subjects who were used in both the schizophrenic and major depressive disorder studies, separate tissue sections were processed with each of the two matched subjects. The parvalbumin antibody (SWant, Bellinzona, Switzerland), a mouse monoclonal immunoglobin G1 used at a dilution of 1:10,000, has been demonstrated to selectively recognize parvalbumin in immunoblot, immunoprecipitation, and immunocytochemical studies
(21,
22).
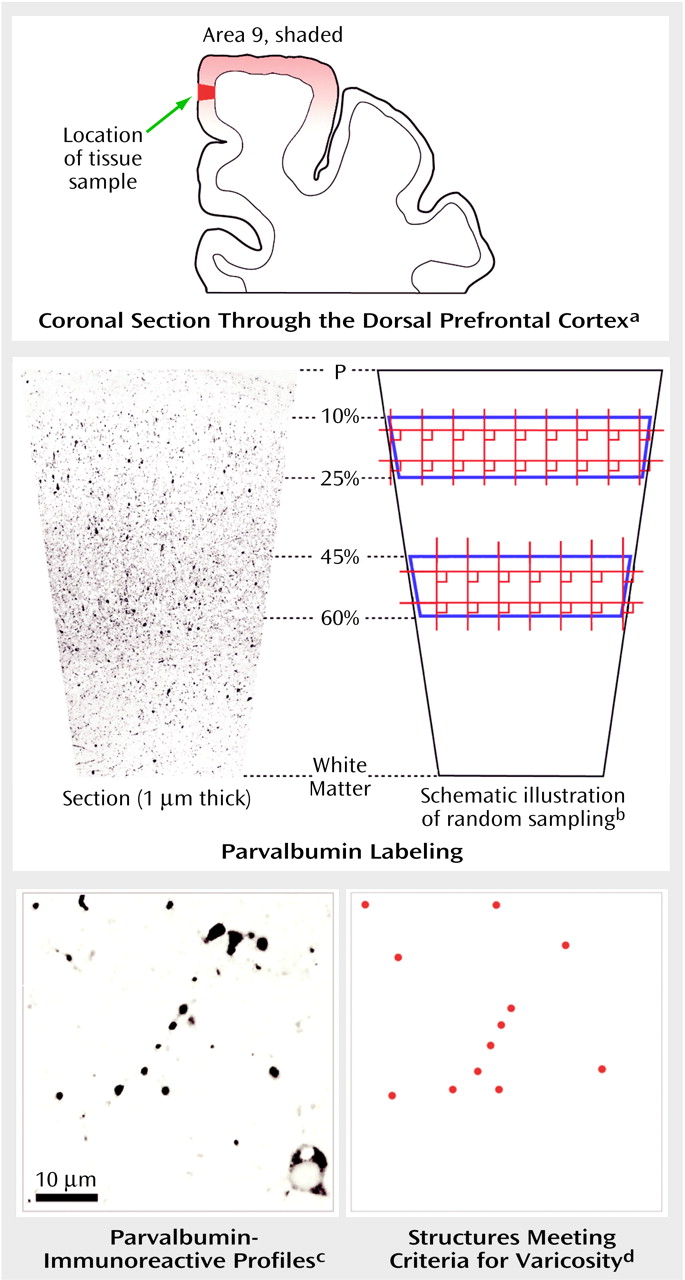
After the tissue was processed for parvalbumin immunoreactivity, the medial portion of area 9 was dissected, postfixed in 2% osmium tetroxide in 0.1 M phosphate buffer, dehydrated through a series of ethanol solutions and propylene oxide, incubated overnight in a 1:1 solution of propylene oxide and Epon (Electron Microscopy Sciences, Fort Washington, Pa.), followed by straight Epon for 2 hours, and then placed between pieces of Aclar plastic for 72 hours at 60°C in order to polymerize the Epon. As seen in
Figure 1, a trapezoid-shaped block, extending from the pial surface to the white matter was removed from the flat-embedded section, cut at 1 μm by using an ultramicrotome, and mounted on slides coded to conceal both subject number and diagnosis. These thin sections permit the detection of parvalbumin-immunoreactive varicosities by eliminating overlying structures.
Quantification of Parvalbumin-Immunoreactive Varicosities
The first section of each block that contained all tissue and no Epon was used for quantification. Cortical thickness was determined by measuring the distance from the pial surface to the white matter for each section. For each subject, the superficial zone (corresponding to cortical layers 2 and superficial 3) and middle zone (corresponding to cortical layers deep 3 and 4) were defined as 10%–25% and 45%–60% of the depth from the pial surface, respectively, as previously described
(23,
24). Stereo Investigator software (MicroBrightField, Inc., Colchester, Vt.) was used to draw contours around the zones of interest on the basis of these percentages. The fractionator method with a 100× oil immersion objective (NA 1.3) was used to systematically and randomly sample by placing a series of sampling grids (150 μm by 150 μm) over each contour. Each sampling grid contained a single counting frame (30 μm by 30 μm) with designated inclusion and exclusion boundaries. In each counting frame, parvalbumin-immunoreactive varicosities were identified by using criteria (round, intensely immunoreactive, and <1.5 μm in diameter) derived from our previous light and electron microscopic studies of parvalbumin immunoreactivity in human and monkey prefrontal cortex
(16). These sampling parameters resulted in mean coefficients of error across all subjects of 0.11 (SD=0.04) in the middle zone and 0.16 (SD=0.05) in the superficial zone. More than 20,000 parvalbumin-immunoreactive varicosities were sampled in this study, with a mean of 254.4 varicosities counted per subject (SD=111.3).
All counts were made by one investigator (D.A.C.) who was blind to subject number and diagnosis. The intrarater reliability of varicosity counts within a section was confirmed by an intraclass correlation coefficient (ICC) of 0.97 (95% confidence interval [CI]=0.76–1.00). The consistency of counts between raters (D.A.C. and J.N.P.) within sections, and by one rater (D.A.C.) across sections within subjects, were also assessed. In this study, the interrater ICC was 0.81 (95% CI=0.44–0.93), and the between-section, within-subject ICC was 0.93 (95% CI=0.71–0.98).
In order to determine the extent to which the parvalbumin-immunoreactive varicosities represented axon terminals, we conducted dual-label fluorescence, confocal microscopy studies in three normal comparison subjects. Semiquantitative assessments in the middle cortical zone revealed that most of the parvalbumin-immunoreactive structures meeting the morphological criteria for a varicosity were also immunoreactive for synaptophysin, a synaptic vesicle protein localized to axon terminals
(25). In contrast, only approximately half of the parvalbumin-immunoreactive varicosities were labeled for GABA transporter-1 (GAT-1), a specific marker of GABA axon terminals (data not shown). Together these findings confirm that most of the structures identified as parvalbumin-immunoreactive varicosities were axon terminals and that in the middle cortical layers, approximately half of these were likely to represent thalamic axon terminals.
Electron Microscopy Studies
In order to verify the results of the light microscopic studies, we also examined brain specimens from two additional male subjects for whom the relatively short postmortem interval and the tissue fixation (immersed in 4% paraformaldehyde and 0.2% glutaraldehyde in 0.1 M, phosphate buffer, pH 7.4, for 24 hours at room temperature followed by 24 hours at 4°C) made electron microscopic studies possible. One subject had no known history of psychiatric or neurologic disorders (age=19 years; postmortem interval=7.0 hours), and the other met diagnostic criteria for schizoaffective disorder (age=25 years; postmortem interval=5.0 hours).
Vibratome sections (50 μm) containing medial prefrontal cortex area 9 were processed for parvalbumin immunoreactivity and prepared for electron microscopy as previously described
(16). Trapezoid blocks, cut from cortical layers 2 and superficial 3 and from layers deep 3 and 4, were sectioned by using an ultramicrotome, and three serial ultrathin sections (80 nm) were collected on copper grids. Grids were counterstained with uranyl acetate and lead citrate and examined on a 100 CX electron microscope (JEOL, Peabody, Mass.). Neuronal elements of relevance to this study were identified by using the criteria of Peters et al.
(26). For each block, 1–2 grids (separated by at least 10 grids) were analyzed. One section per grid was arbitrarily chosen as the starting point for analysis. Within this section, all parvalbumin-immunoreactive axon terminals were identified, photographed at X19,000, and classified as either forming or not forming a synapse. Those terminals forming synaptic contacts were then further divided on the basis of synaptic type. Gray’s type I (excitatory) synapses were identified by the widened and parallel spacing of apposed plasmalemmal surfaces, an accumulation of round synaptic vesicles at the presynaptic membrane, and a thick postsynaptic density. In contrast, Gray’s type II (inhibitory) synapses had thin postsynaptic densities, intercleft filaments, and synaptic vesicles that were usually pleomorphic in shape. All terminals were initially classified by one investigator (D.S.M.), and then a second investigator (D.A.L.) independently reviewed all the micrographs in a random order. The interrater ICC was 0.98 (95% CI=0.98–0.99).
The cross-sectional area (μm
2) of parvalbumin-immunoreactive axon terminals were measured on negatives with a computer digitizing system (Research Imaging Inc., St. Catherines, Ontario), as previously described
(16).
Studies in Haloperidol-Treated Monkeys
To assess the possible influence of psychotropic medications on the density of parvalbumin-immunoreactive varicosities, we studied four male cynomolgus (
Macaca fascicularis) monkeys after long-term treatment with haloperidol decanoate (mean dose of 16.0 mg/kg [SD=2.1] administered every 4 weeks) and benztropine mesylate (1 mg twice daily), as previously described
(27). Each drug-treated animal was matched to an untreated comparison animal for sex, age, and weight. Trough serum levels (ng/ml) of haloperidol (mean=4.3, SD=1.1) were within the range shown to be therapeutic in humans
(28). Following 9–12 months of treatment, both animals in a matched pair were euthanized with an overdose of pentobarbital, and the brains were removed. All procedures were approved by the University of Pittsburgh’s Institutional Animal Care and Use Committee.
Following a 45-minute postmortem interval, coronal tissue blocks from the left hemisphere were prepared and processed for parvalbumin immunocytochemistry in a manner identical to that described for the human subjects. The densities of parvalbumin-immunoreactive varicosities in the superficial and middle cortical zones of medial area 9 were determined in an identical fashion to that previously described with one exception. Because of the thinner cortical mantle in monkeys, the sampling (100 μm by 100 μm) and counting frames (25 μm by 25 μm) were smaller in size. The coefficient of error in each sampling zone of each animal was <0.1.
Statistical Analyses
Paired t tests were used to compare schizophrenic and comparison subjects on demographic variables. The distribution of parvalbumin-immunoreactive varicosity density measures was normalized by using a log10 transformation, and between-group comparisons were made by using analysis of covariance (ANCOVA). For each of the two laminar zones, the density of parvalbumin-immunoreactive varicosities was the dependent variable with diagnostic group as the main effect, and age, sex, postmortem interval, and tissue storage time as covariates. Since a separate analysis with diagnostic group as the main effect, pair as a blocking variable, and tissue storage time as a covariate produced the same results, only the first analysis is reported. In addition, the influence of sex, substance abuse, cause of death, and antipsychotic medication status at the time of death on the differences in parvalbumin-immunoreactive varicosity density within subject pairs was assessed by using ANCOVA, with age, postmortem interval, and tissue storage time of the subjects with schizophrenia or major depression as covariates. To explore the effects of age at onset and duration of illness on parvalbumin-immunoreactive varicosity density in the subjects with schizophrenia, we used multiple regression analyses with age at time of death included as an independent variable. In the monkey study, an ANOVA was conducted with the density of parvalbumin-immunoreactive varicosities as the dependent variable and drug treatment group as the main effect.
Results
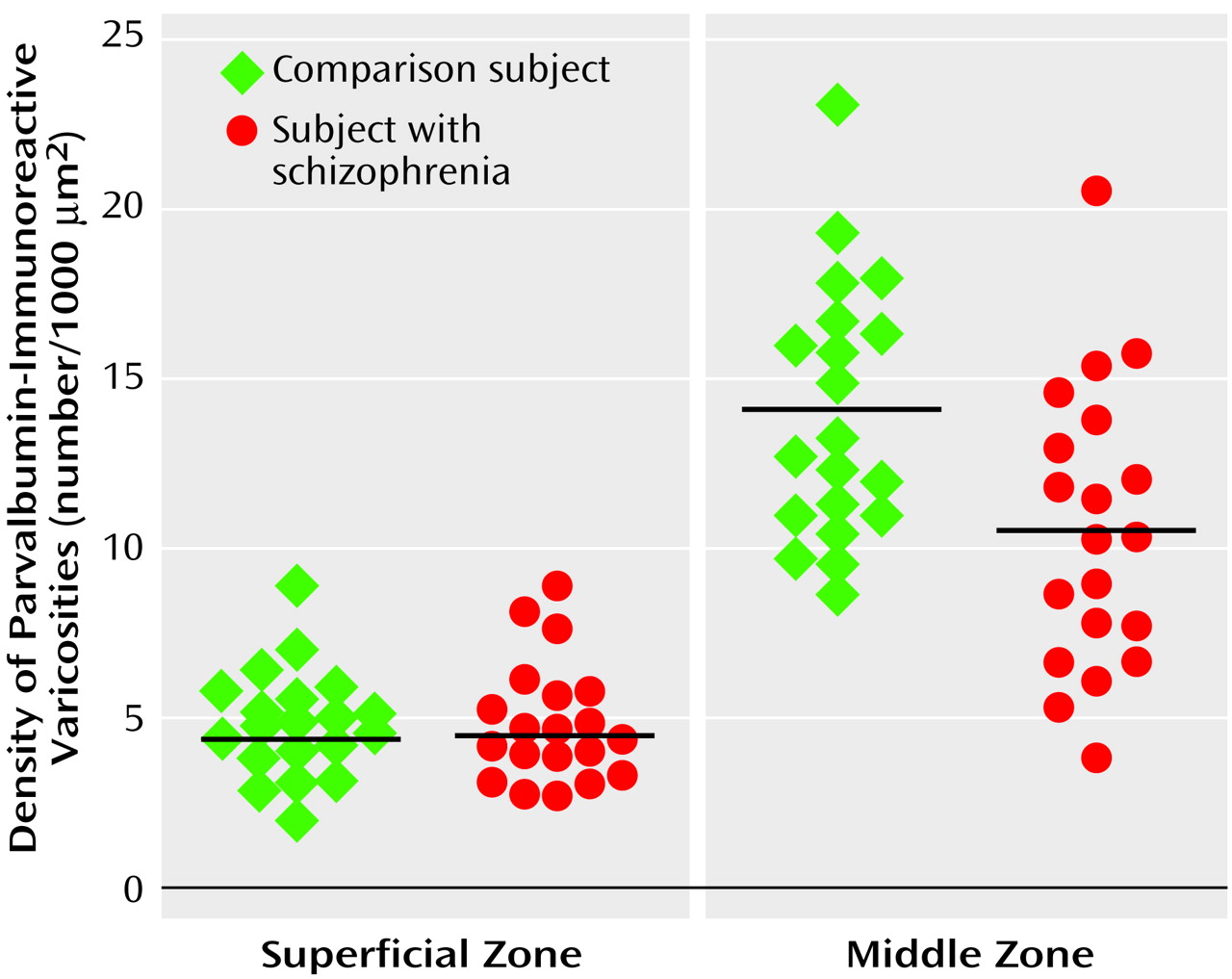
As shown in
Figure 2, the relative densities (per 1000 μm
2) of parvalbumin-immunoreactive varicosities in the superficial zone of prefrontal cortex area 9 were virtually identical in the comparison (mean=4.8, SD=1.6) and schizophrenic (mean=4.9, SD=1.8) subjects. In contrast, in the middle zone, the density (per 1000 μm
2) of parvalbumin-immunoreactive varicosities in the schizophrenic subjects (mean=10.6, SD=4.2) was significantly lower than that of the comparison subjects (mean=14.0, SD=3.8) (24% difference; F=8.71, df=1, 34, p=0.006). Repeated measures analysis confirmed a significant diagnosis-by-layer interaction in varicosity density (F=5.8, df=1, 34, p=0.02). The difference between subject groups in the middle zone could not be attributed to effects of sex (F=1.66, df=1, 34, p=0.21), age (F=0.11, df=1, 34, p=0.74), postmortem interval (F=0.10, df=1, 34, p=0.76), or tissue storage time (F=0.61, df=1, 34, p=0.44). Analyses excluding the three schizophrenic subjects who had neuropathological abnormalities and their matched comparison subjects also showed no difference across subject groups in parvalbumin-immunoreactive varicosity densities in the superficial zone, but varicosity density in the middle zone remained significantly lower in the schizophrenic subjects (F=4.71, df=1, 28, p=0.04). As shown in
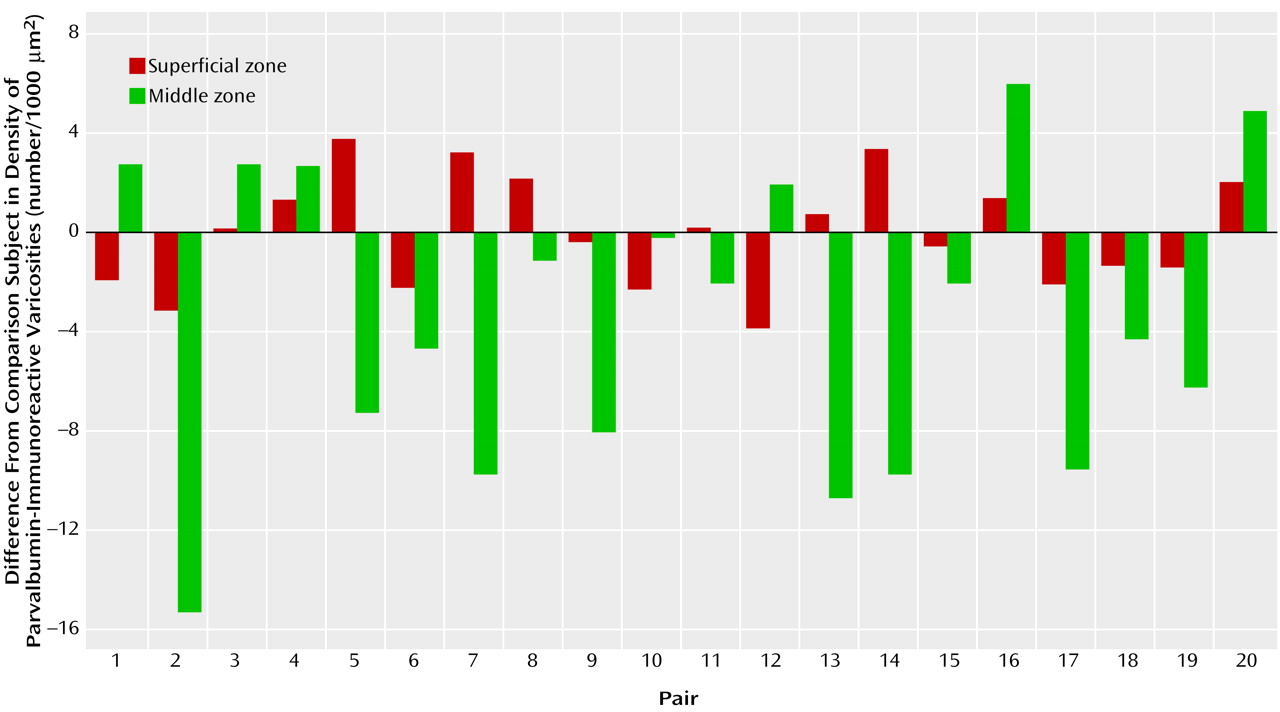
Figure 3, 14 of the 20 schizophrenic subjects had a lower density of parvalbumin-immunoreactive varicosities in the middle zone relative to their matched comparison subjects, with this difference exceeding 15% in 12 of the subject pairs.
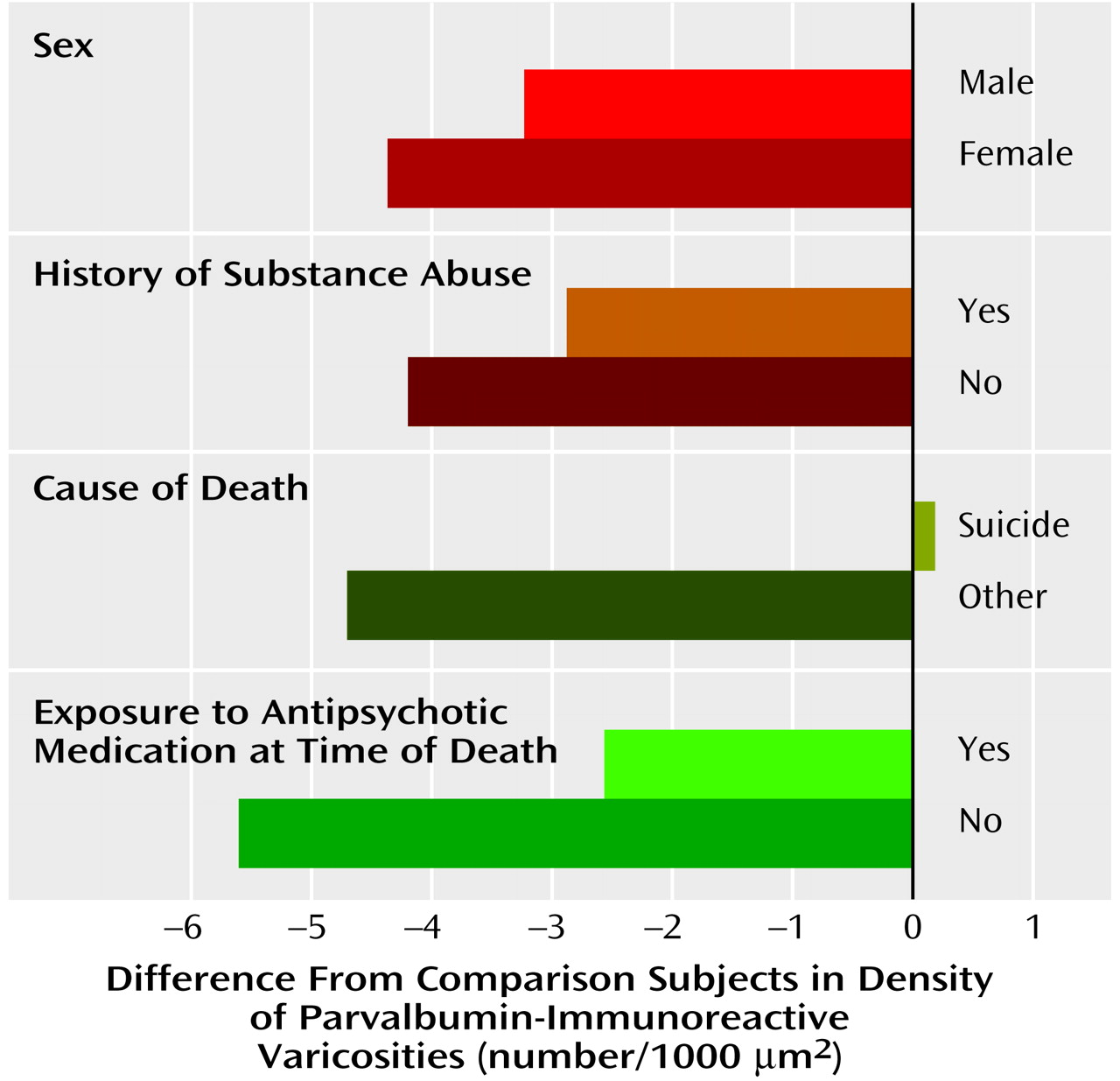
In the middle zone, the differences in density of parvalbumin-immunoreactive varicosities within the matched pairs of schizophrenic and comparison subjects did not vary as a function of sex (F=1.14, df=1, 15, p=0.30), death by suicide (F=0.83, df=1, 15, p=0.38), history of substance abuse (F=0.14, df=1, 15, p=0.72), or presence of antipsychotic medications at the time of death (F=2.0, df=1, 15, p=0.18) (
Figure 4). In addition, neither age at onset (r=0.09, df=18, p=0.71) nor duration of illness (r=0.31, df=18, p=0.20) was associated with the density of parvalbumin-immunoreactive varicosities in the middle zone of schizophrenic subjects.
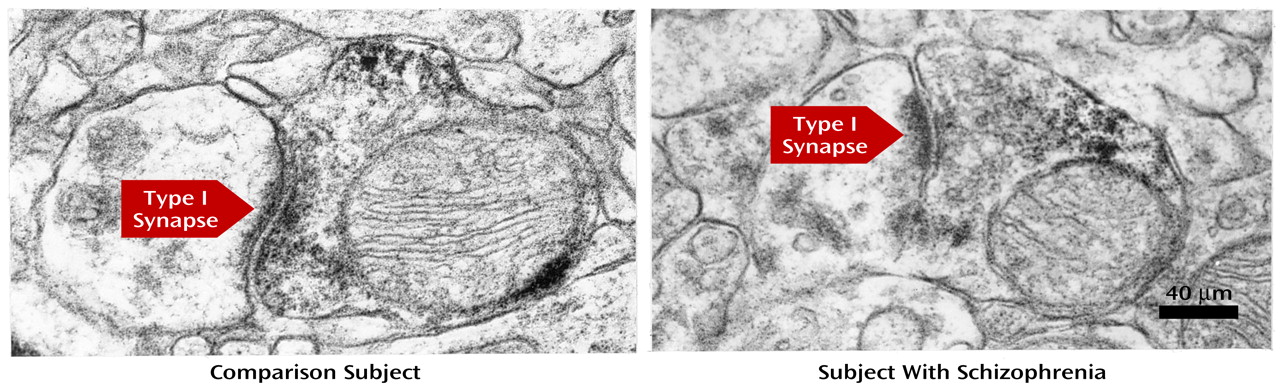
In order to further assess the specificity of these observations for thalamic axon terminals, we examined an additional matched pair of subjects for which the postmortem interval and fixation conditions were appropriate for electron microscopy (see Method section). On the basis of our previous observations in monkey prefrontal cortex
(16), we expected to find parvalbumin-immunoreactive axon terminals forming exclusively type II (i.e., from intrinsic GABA neurons) synapses in the superficial zone and roughly equal proportions of type II and type I (i.e., from the thalamus) synapses in the middle zone of human prefrontal cortex area 9. However, in the comparison subject, no parvalbumin-immunoreactive axon terminals (out of 65) formed identifiable type II synapses in either zone, and in the middle zone, 30% (N=10 of 33) of the parvalbumin-immunoreactive axon terminals formed type I synapses (
Figure 5). Thus, although synaptic vesicles and membrane appositions were readily evident in parvalbumin-immunoreactive axon terminals, the thin postsynaptic densities and intercleft filaments characteristic of type II synapses
(29) did not appear to be preserved in our material. This interpretation is supported by the observation that the cross-sectional area (μm
2) of parvalbumin-immunoreactive axon terminals in the superficial zone (mean=0.365, SD=0.146) was 27% smaller than those in the middle zone (mean=0.502, SD=0.169), similar to our finding in monkey prefrontal cortex that parvalbumin-immunoreactive axon terminals forming type II synapses are smaller than those forming type I synapses
(16). On the basis of these findings, we predicted that the proportion of parvalbumin-immunoreactive varicosities in the middle zone that formed type I synapses would be lower in the subject with schizophrenia. Consistent with this prediction, only 11.5% (N=3 of 26) of the sampled parvalbumin-immunoreactive axon terminals formed type I synapses in the schizophrenic subject compared to 30% (N=10 of 33) in the comparison subject.
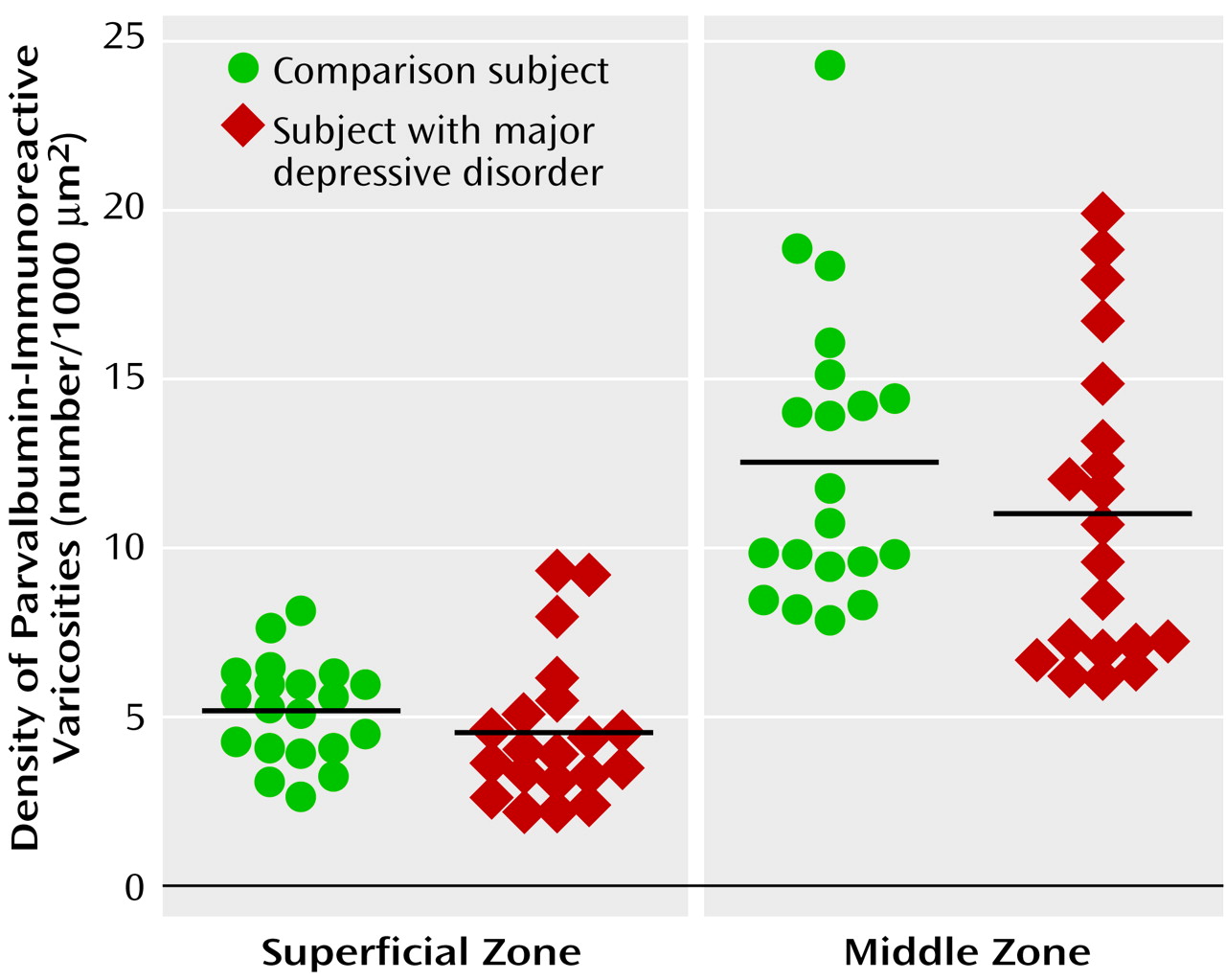
The diagnostic specificity of the lower density of parvalbumin-immunoreactive varicosities in the middle zone of subjects with schizophrenia was tested by determining parvalbumin-immunoreactive varicosity density in subjects with major depression. Mean density was 12% lower in both the superficial (F=1.75, df=1, 34, p=0.20) and middle (F=1.28, df=1, 34, p=0.27) zones in the subjects with major depression relative to their matched comparison subjects (
Figure 6). In addition, in the middle zone, the differences in density within the matched pairs of major depressive and comparison subjects did not vary as a function of history of psychosis (F=0.43, df=1, 15, p=0.52), history of exposure to antipsychotic medications (F=0.47, df=1, 15, p=0.50), or death by suicide (F=1.7, df=1, 15, p=0.21).
In order to determine the possible influence of antipsychotic medications on the measures of parvalbumin-immunoreactive varicosity density, we conducted similar studies in monkeys after 9–12 months of haloperidol treatment. In contrast to the findings in schizophrenia, parvalbumin-immunoreactive varicosity density (per 1000 μm2) in the middle zone did not differ between the comparison (mean=11.4, SD=2.4) and haloperidol-treated (mean=10.9, SD=1.3) monkeys (F=0.137, df=1, 6, p=0.72).
Discussion
In prefrontal cortex area 9, schizophrenia appears to be associated with a lower density of parvalbumin-immunoreactive varicosities that is specific to the middle cortical layers, the termination zone of axonal projections from the mediodorsal thalamic nucleus
(9). This alteration may be specific to the pathophysiology of schizophrenia, since it was not observed in subjects with major depressive disorder or in monkeys after 9–12 months of haloperidol treatment.
Studies in nonhuman primates have demonstrated that parvalbumin is present in cortically projecting thalamic neurons
(15). In addition, in both monkey and human prefrontal cortex, differences among parvalbumin-immunoreactive axon terminals in laminar distribution, type of synaptic specialization, predominant postsynaptic target, and size indicate that they comprise two distinct populations: 1) the terminals of a subset of intrinsic cortical GABA neurons and 2) the terminals of projections from the thalamus
(16). Parvalbumin-immunoreactive terminals of intrinsic GABA neurons form exclusively type II synapses and are found across cortical layers, whereas parvalbumin-immunoreactive terminals from the mediodorsal thalamic nucleus form only type I synapses that are restricted to the middle cortical layers. Thus, our findings of 1) a significant deficit in the density of parvalbumin-immunoreactive varicosities specific to the middle cortical layers of prefrontal cortex area 9 in subjects with schizophrenia, and 2) a decrease in the proportion of parvalbumin-immunoreactive axon terminals with type I synapses in the middle cortical layers in the electron microscopy case study are both consistent with an alteration in the mediodorsal thalamic nucleus projection to the prefrontal cortex. This interpretation is supported by the observation that neither the density, laminar distribution, nor size of parvalbumin-immunoreactive cortical neurons were lower in prefrontal cortex area 9 in a previous study
(19) that included a subset of the subjects with schizophrenia examined in the present study. (However, a preliminary report from another group did describe a deficit of parvalbumin-immunoreactive neurons in area 9 in schizophrenia
[30].) Furthermore, changes in the expression of two markers of GABA neurotransmission, glutamate decarboxylase and the GABA membrane transporter (GAT-1), have been observed in both the superficial and middle layers of the prefrontal cortex in subjects with schizophrenia
(23,
24,
31–
33). Thus, these data suggest that the observed laminar-specific deficit in parvalbumin-immunoreactive varicosities is unlikely to be attributable to changes in axon terminals arising from intrinsic GABA neurons. However, an alteration in the axon terminals of parvalbumin-containing cortical GABA neurons that is restricted to the middle layers cannot be directly excluded.
Both the electron microscopy data and the extensive colocalization of synaptophysin and parvalbumin immunoreactivities in varicosities support the interpretation that the majority of parvalbumin-immunoreactive varicosities counted in this study represent axon terminals, although certainly some are likely to be parvalbumin-immunoreactive dendritic shafts cut in cross-section. In addition, our previous electron microscopy studies
(16) indicate that approximately 50% of the parvalbumin-immunoreactive axon terminals in the middle cortical layers are not from the mediodorsal thalamic nucleus. Thus, if our findings do reflect a change only in the density of mediodorsal thalamic nucleus terminals, then the magnitude of deficits in these terminals in schizophrenia may actually be greater than the observed mean difference of 24%.
These alterations in parvalbumin-immunoreactive varicosities appear to be related to the disease process of schizophrenia or at least not attributable to coexisting factors such as a history of substance abuse, major depression, or suicide. Although the difference from matched comparison subjects in parvalbumin-immunoreactive varicosity density in subjects who died by suicide compared to other causes was not statistically significant, the five subjects with schizophrenia who died by suicide did not, as a group, show a reduction in parvalbumin-immunoreactive varicosities relative to comparison subjects (
Figure 4). However, among the depressed subjects, suicide was associated with a greater deficit in parvalbumin-immunoreactive varicosities in the middle layers, although again the difference relative to subjects with other causes of death was not significant (data not shown).
The lower density of parvalbumin-immunoreactive varicosities in the middle zone of the prefrontal cortex also does not appear to be due to treatment with antipsychotic medications. For example, the six subjects with schizophrenia who were not receiving antipsychotic medications at the time of death actually had a larger deficit in parvalbumin-immunoreactive varicosities than did the 14 subjects who were receiving antipsychotic medications at the time of death (
Figure 4). In addition, subject 234, who had never been treated with antipsychotic medications, had one of the largest deficits in parvalbumin-immunoreactive varicosity density (pair 7 in
Figure 3>). Finally, the studies of haloperidol-treated monkeys also indicate that antipsychotic medications do not decrease parvalbumin-immunoreactive varicosities in the thalamic recipient zone of the prefrontal cortex.
Although other interpretations cannot be completely excluded, the results of this investigation converge with a growing body of literature indicating that disturbances in mediodorsal thalamic nucleus-prefrontal cortex circuitry may play a critical role in the pathophysiology of schizophrenia. Imaging studies have revealed that the thalamus has a smaller volume in subjects with chronic as well as first-episode schizophrenia
(10,
11), less metabolic activity
(11,
34), and lower concentrations of
N-acetylaspartate, a putative marker of neuronal/axonal integrity
(35,
36). Postmortem studies have described deficits in mediodorsal thalamic nucleus neuron number and volume
(12–
14), as well as a lower density of parvalbumin-immunoreactive neurons in the anterior nucleus (which also furnishes projections to the prefrontal cortex)
(37).
The present study suggests that these disturbances within the thalamus are associated with fewer markers of thalamic axon terminals in the prefrontal cortex, although whether these terminals are fewer in number or have undetectable levels of parvalbumin cannot be determined. However, previous studies have reported alterations in the prefrontal cortex that are consistent with a lower number of projections from the mediodorsal thalamic nucleus. For example, the density of dendritic spines, a principal synaptic target of thalamic projections, is lower in the dorsal prefrontal cortex of subjects with schizophrenia
(38,
39), and these changes appear to be most marked on the basilar dendrites of deep layer 3 pyramidal cells, which extend through the termination zone of thalamic inputs in layers deep 3 and 4
(39). The somal size of deep layer 3 pyramidal neurons is also smaller in schizophrenia
(40,
41), a change that may occur with a loss of afferent inputs
(42). In addition, the observed alterations in prefrontal cortex GABA neurons in schizophrenia
(23,
24,
31–
33) may reflect activity-dependent changes secondary to lower thalamic drive (see references
43 and
44 for reviews).
Thus, convergent lines of evidence support the view that schizophrenia is associated with disturbances in thalamo-prefrontal connectivity. Given the critical role of thalamic inputs to prefrontal cortex function
(45), these disturbances are likely to contribute to the dysfunction of the dorsal prefrontal cortex and the cognitive symptoms that are present in schizophrenia. However, as discussed elsewhere
(43), additional studies are needed to determine whether the prefrontal cortex abnormalities are secondary to those in the thalamus, or whether the thalamic abnormalities are the consequence of a more primary disturbance in the prefrontal cortex.