In the last decade, firm evidence has emerged suggesting that certain derivatives of progesterone, so-called “neuroactive steroids,” may modulate neuronal excitability through rapid nongenomic effects at the cell surface
(1,
2). The 3α-reduced metabolites of progesterone—such as 3α,5α-tetrahydroprogesterone (3α,5α-THP, allopregnanolone) and 3α,5β-THP (pregnanolone)—are potent positive allosteric modulators of the γ-aminobutyric acid
A (GABA
A) receptor, while the stereoisomer 3β,5α-THP may act as a functional antagonist for GABA-agonistic steroids
(1,
2).
Progesterone is formed from pregnenolone by the 3β-hydroxysteroid dehydrogenase/Δ
5-Δ
4-isomerase. The 5α-reductase and the 5β-reductase catalyze the reduction of progesterone into the 5α-pregnane steroids 5α-dihydroprogesterone (5α-DHP) and 5β-DHP, which may be further reduced to the neuroactive steroids 3α,5α-THP and 3α,5β-THP by the 3α-hydroxysteroid oxidoreductase
(1,
2). Although animal studies suggest a pronounced anxiolytic activity of these 3α-reduced neuroactive steroids
(1,
2), to our knowledge no data on their role in panic disorder and panic disorder treatment are available. We therefore studied baseline concentrations of GABA
A receptor-modulating neuroactive steroids in patients with panic disorder and in matched healthy comparison subjects and monitored the effects of 24 weeks of paroxetine treatment in the panic disorder patients.
Method
Ten patients (seven women and three men; mean age=37.2 years, SD=10.2) with a diagnosis of panic disorder but without a comorbid axis I disorder according to the Structured Clinical Interview for DSM-IV were studied. The mean age at onset of panic disorder was 24.1 years (SD=6.6). Two patients had also participated in a previous challenge study
(3). Baseline scores on the Panic and Agoraphobia Scale
(4) indicated a moderate severity of panic and agoraphobia (mean=23.0, SD=8.3). The mean Hamilton Depression Rating Scale
(5) score was 8.8 (SD=3.1), and the mean Hamilton Anxiety Rating Scale
(5) score was 14.2 (SD=3.1). Clinical Global Impression rating
(5) indicated mild to moderate impairment (mean=5.0, SD=1.0). Mean panic attack frequency per week was 2.0 (SD=1.8). Ten healthy age- and sex-matched subjects with no personal or family history of a psychiatric disorder served as comparison subjects. After blood sampling on day 0, each panic disorder patient was treated with an increasing dose of paroxetine every morning for 24 weeks. The paroxetine dose was increased by 10 mg/day each week. If treatment increased anxiety or arousal, dose increase was delayed until the worsening disappeared. Maximal clinical effectiveness and minimal side effects were achieved with a mean paroxetine dose of 37.0 mg/day (SD=11.6). Patients did not receive any additional psychopharmacological treatment during the study. Subjects had been medication free for at least 10 days and had undergone a thorough medical examination to rule out other illnesses, drug intake, or lifestyles that could interfere with the study.
Plasma samples were obtained after written informed consent at 10:00 a.m. on day 0, day 14, and day 28 and then every 4 weeks until week 24. They were quantified for progesterone, 5α-DHP, 5β-DHP, 3α,5α-THP, 3α,5β-THP, and 3β,5α-THP by means of a highly sensitive combined gas chromatography/mass spectrometry analysis
(6). A Finningham Trace gas chromatography/mass spectrometry unit equipped with a capillary column was used to analyze the derivatized steroids in the negative ion chemical ionization mode. The detection limit was approximately 10 fmol.
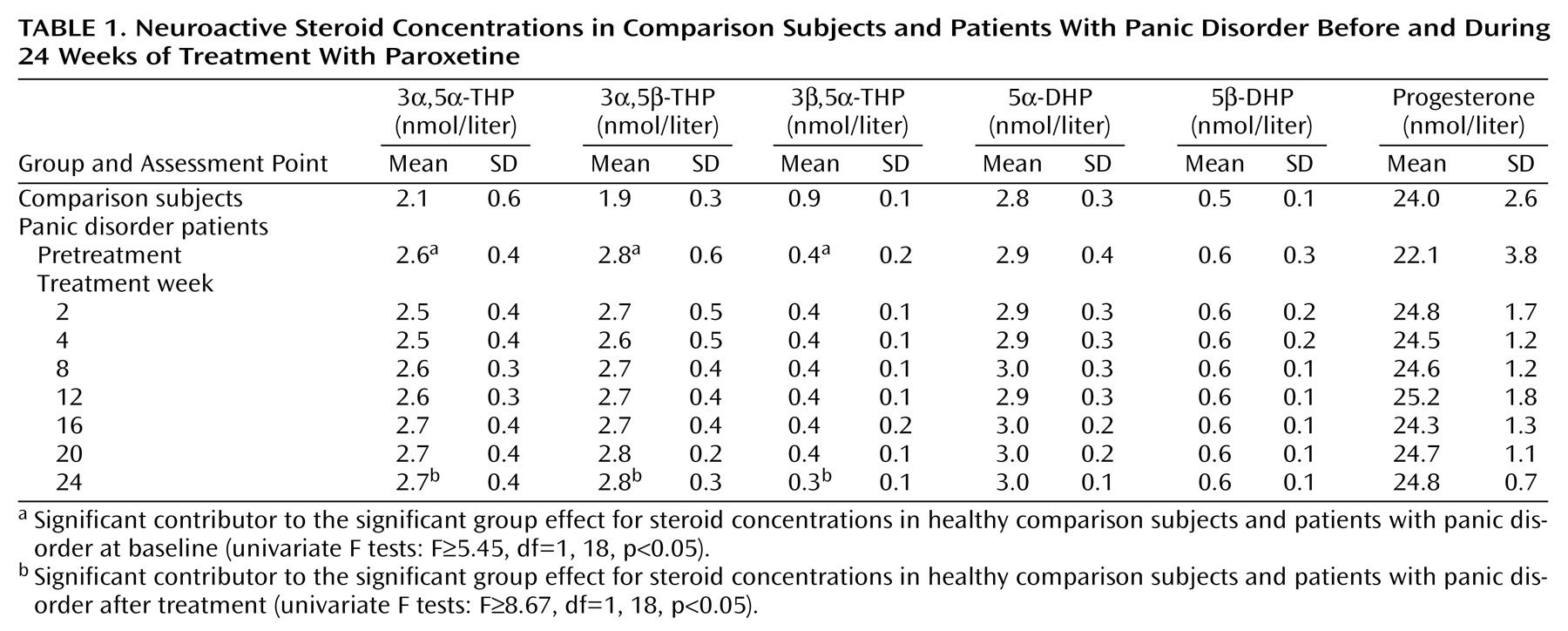
The group effect on steroid concentrations, i.e., differences between healthy comparison subjects and panic disorder patients at day 0, as well as at week 24 after treatment with paroxetine, were tested for significance with Wilks’s multivariate test by applying in each case a one-factorial multivariate analysis of variance (MANOVA) with group as a between-subjects factor. Where there was a significant group effect, the identification of steroids that contributed significantly to this effect was carried out by subsequent univariate F tests. Possible differences in steroid concentrations during paroxetine treatment were tested for significance by using a one-factorial repeated measures analysis of variance with time as the within-subjects factor with eight levels for each steroid. Tests with contrasts were subsequently performed with comparisons to day 0. To approach normality and to increase homogeneity of the data, the variables were transformed by log transformation (x*=lg10[x]) before analysis. As a nominal level of significance, alpha=0.05 was accepted. According to the Bonferroni procedure, all post hoc tests were performed at a reduced level of significance.
Results
In the comparison of neuroactive steroid concentrations in panic disorder patients and comparison subjects at day 0, MANOVA revealed a significant group effect (F=5.53, df=6, 13, p=0.005) that was attributable to the concentrations of 3α,5α-THP, 3α,5β-THP, and 3β,5α-THP (univariate F tests: F≥5.45, df=1, 18, p<0.05) but not to those of progesterone, 5α-DHP, or 5β-DHP (
Table 1).
All panic disorder patients experienced improvement after 24 weeks of paroxetine treatment. Clinical Global Impression and Panic and Agoraphobia Scale scores declined to 1.6 (SD=0.5) and 3.1 (SD=3.7), respectively. The clinical effectiveness of paroxetine treatment was supported by a decrease in scores on the Hamilton depression (mean=1.4, SD=0.9) and anxiety (mean=2.9, SD=1.2) scales. Mean panic attack frequency after treatment was 0.2 (SD=0.4) per week. However, the concentrations of the six neuroactive steroids did not change during the treatment with paroxetine (Wilks multivariate F=0.22–3.51, df=7, 3, all p≥0.10). After treatment, MANOVA still revealed a significant group effect (F=7.78, df=6, 13, p=0.001), again attributable to the concentrations of 3α,5α-THP, 3α,5β-THP, and 3β,5α-THP (univariate F tests: F≥8.67, df=1, 18, p<0.05).
Discussion
Relative to matched healthy comparison subjects, patients with panic disorder had greater plasma concentrations of the GABA-agonistic modulators 3α,5α-THP and 3α,5β-THP and a concomitant lower plasma concentration of 3β,5α-THP, which may act as a functional antagonist for GABA-agonistic steroids
(1,
2). The precursors progesterone, 5α-DHP, and 5β-DHP were comparable in patients with panic disorder and the comparison subjects. Effective panic disorder treatment with paroxetine produced no further changes in neuroactive steroid concentrations. The size of our study limits the statistical power to detect clear changes, such as those observed in patients with major depression
(6–
8).
Our findings in panic disorder patients were the opposite of those seen in patients with major depression, whose lower levels of 3α,5α-THP and 3α,5β-THP and higher concentrations of 3β,5α-THP could be normalized by treatment with antidepressants
(6–
8). Since selective serotonin reuptake inhibitors shift the activity of 3α-hydroxysteroid oxidoreductase in the reductive direction
(9), the ineffectiveness of paroxetine treatment on the concentrations of neuroactive steroids in panic disorder patients may be attributed in part to already increased baseline concentrations. The observed differences in neuroactive steroid composition in patients with panic disorder, which may result in greater GABA
A receptor-mediated neuronal activity, are an unexpected finding. However, the reduced sensitivity to benzodiazepines of patients with panic disorder
(10) might be related to these differences in GABA
A receptor-modulating neuroactive steroid composition. Since experimentally induced panic attacks in patients with panic disorder are accompanied by pronounced decreases in the concentrations of 3α,5α-THP and 3α,5β-THP and a concomitant increase in the concentration of 3β,5α-THP
(3), it may be hypothesized that the alterations in neuroactive steroid composition reflect compensatory mechanisms against the occurrence of spontaneous panic attacks.
Our results provide to our knowledge the first evidence for distinct changes in GABAA receptor-modulating neuroactive steroids in patients with panic disorder and major depression. When attempting to pharmacologically modify the equilibrium of neuroactive steroids as a treatment for psychiatric disorders, consideration should also be given to baseline concentrations and possible counterregulatory mechanisms.