The development of posttraumatic stress disorder (PTSD) in a significant minority of people exposed to trauma and the high morbidity and treatment resistance that can occur after the disorder has become established
(1) have stimulated interest in understanding the early stages of its pathogenesis. Dissociation
(2,
3) and a faster heart rate
(4) proximate to the trauma have been found to predict subsequent PTSD. Peritraumatic dissociation has been interpreted as relating to a more fragmented acquisition of trauma memory that remains unintegrated in memory storage
(2). A faster heart rate has been linked to enhanced consolidation of trauma memory mediated by sympathetic nervous system activation
(4).
While measures of initial trauma reactions have accounted for significant variance in predicting PTSD, processes that occur during the first 1 to 2 months after trauma appear to be important determinants of the progression or resolution of posttraumatic distress. In a prospective study of rape victims
(5), the percent of the cohort that met symptom criteria for PTSD dropped from 94% within 2 weeks of the assault to 41% 8 weeks later, after which the rate of PTSD did not diminish
(5). A report from the same study
(6), in which higher initial heart rate was associated with subsequent PTSD, indicated that a greater heart rate response to a startling stimulus was present in the group developing PTSD at 1 and 4 months but not at 1 week after trauma exposure. The latter finding was suggested to support the occurrence of a progressive sensitization of the CNS during the month after trauma exposure
(6).
There are a number of reasons to suspect that sleep has an important influence on the regulation of arousal and the processing of traumatic memory during the acute period after trauma exposure. Sleep is a restorative state of diminished arousal. Disruption of sleep can induce tension, vigilance, and irritability
(7), which are also features of PTSD. REM sleep, the sleep stage most specifically associated with dreaming
(8), has been postulated to have a role in integrating traumatic and other distressing memories
(9–
11). Experimental studies
(12,
13) have provided evidence for a role for REM (and other sleep stages) in consolidating recently acquired memories. The evidence that REM sleep has a specific role in processing emotional memory includes the emotional content of many REM dreams
(8) and an experimental finding relating recall of words with negative emotional content to intervening REM sleep
(14). The potential for REM sleep to facilitate adaptive processing of distressing emotions is supported by findings that earlier onset of REM sleep and higher dream activity in the initial REM period predicted greater reduction of the depressive symptoms that were induced by a disturbing life event
(11).
The DSM-IV criteria for PTSD include trauma-related nightmares and difficulty initiating and maintaining sleep. Despite the prominence of these symptoms, polysomnographic studies of chronic PTSD have not generated a clear characterization of sleep abnormalities in the disorder. Some study findings have suggested shortened or disrupted sleep
(15–
17), whereas others have not found reduced sleep efficiency or sleep time
(18,
19). Two studies
(16,
18) have reported greater REM density (frequency of eye movements during REM sleep) with PTSD. Ross et al.
(20) also found greater motor activity during REM sleep, and our group reported a tendency for anxious awakenings with and without dream recall to have been preceded by REM sleep
(21). It has been suggested that these findings implicate greater phasic-event generation
(18) and occurrences of disrupted REM continuity
(21) in patients with PTSD.
These studies featured PTSD in male combat veterans, typically decades after the onset of the disorder. There are likely a number of secondary factors that evolve to affect sleep when PTSD persists for many years. The only report that we were able to identify in which polysomnographic measures were obtained acutely after trauma exposure
(22) featured three patients who were hospitalized for “acute combat fatigue.” Their sleep was reported to have been of short duration, fragmented, and high in motor activity. REM periods were described to have been “rare and short.” Neither comparison groups nor follow-up information were included in this unique preliminary report
(22).
Subjects in the present study were recruited while hospitalized for injuries from life-threatening events and underwent polysomnographic recording within a month of the trauma. The general aim of this study was to contrast polysomnographic measures among the injured groups who were or were not manifesting PTSD symptoms at follow-up and a group of noninjured comparison subjects. In view of suggested alterations with chronic PTSD, preliminary observation of diminished REM sleep with acute combat fatigue, and possible adaptive functions in the processing of emotional memory, we were especially interested in the characteristics of REM sleep. We hypothesized that disrupted REM sleep would be associated with the development of PTSD.
Method
Subjects
Patients were recruited from the level I trauma center that serves Miami-Dade County, Fla., except for the final four injured subjects, who were recruited from the level I trauma service affiliated with Dartmouth Medical School in New Hampshire after relocation of the principal investigator. A total of 104 patients who were admitted for traumatic injuries and met initial screening criteria signed consent forms for participation in the general study after receiving a full explanation of the purpose and methods of its components. The initial inclusion criteria included having memory of the traumatic incident; having reacted to the experience with fear, helplessness, or horror; and having been conscious and alert on arrival to the trauma center. Additional initial criteria were unimpaired orientation and recall, absence of postconcussive symptoms, no clinical or laboratory evidence for intoxication, absence of radiological signs of CNS injury, and not meeting criteria for a psychiatric disorder in the 6 months preceding admission to the trauma center.
Patients were recruited for polysomnographic evaluation within a month of injury, if by that time they had discontinued narcotic analgesic medication and endorsed that their pain was not significantly interfering with sleep. These subjects also had no histories or signs suggesting primary sleep pathology (e.g., hypersomnolence, obesity, loud snoring). Continuing use of oral narcotics and difficulty returning to the hospital setting (for those not eligible before discharge) were the most common reasons for not administering polysomnographic recordings to the patients who met the initial screening criteria. The final group of 21 injured, trauma-exposed subjects who underwent polysomnographic recordings had a mean age of 35.4 years (SD=9.5); the group included five women (24%), 11 Hispanics (52%), seven non-Hispanic whites (33%), and three non-Hispanic blacks (14%). Thirteen (62%) had been in motor vehicle accidents, two (10%) had been in industrial accidents, and six (29%) had gunshot wounds from assaults of an impersonal nature (e.g., robbery). Principal injuries were internal abdominal in 13 of the patients (62%) and thoracic in three (14%); three others (14%) had bone fractures, and two patients (10%) had partial limb amputations.
In order to evaluate the effects of trauma and injury on sleep in addition to associations of sleep measures with the development of PTSD, 10 healthy noninjured comparison subjects were also recruited for polysomnographic recording (three women; three Hispanics, six whites, one black; mean age=35.4 years, SD=12.0). These subjects were screened to not have significant medical problems, more than infrequent use of alcohol, more than moderate consumption of caffeine, active medication use, and a history of psychiatric episodes in the previous 12 months.
Assessments
Assessments were conducted in Spanish if that was the subject’s preferred language. Initial criteria for being alert and responsive were determined by reviewing Glasgow Coma Scale
(23) scores. Orientation and recall were evaluated with the Galveston Orientation and Amnesia Test
(24). Psychiatric exclusion and trauma-incident psychiatric disorder criteria were determined by use of the Structured Clinical Interview for DSM-IV (SCID)
(25). PTSD symptoms were rated by the Clinician-Administered PTSD Scale
(26). A Spanish translation of the Clinician-Administered PTSD Scale was developed and validated by the study team. Diagnostic criteria were assigned on the basis of the presence of a symptom of at least moderate severity at the second evaluation; severity ratings were anchored to the week of the assessment
(26). These interviews were all conducted by clinicians (M.D., Ph.D., or Psy.D.), with independent assessment occurring after 100% agreement at the level of diagnosis had been achieved on five consecutive evaluations. Assessments were initiated during inpatient stays as soon as patients were medically and surgically stable. The initial Clinician-Administered PTSD Scale was repeated if polysomnographic recording was delayed so that all of the initial Clinician-Administered PTSD Scale scores reported here rated symptoms for the week that led up to and included the day of the polysomnographic recording. A follow-up Clinician-Administered PTSD Scale and SCID were conducted by the same interviewer 6 weeks after the initial assessment (approximately 2 months after the trauma), at which time all patients had been discharged from the hospital.
Polysomnographic recordings were obtained on average 17.1 days (SD=8.2) after the traumatic incidents (range=3–30). Recordings were not performed until patients had discontinued narcotic analgesics and other medications affecting CNS function for at least five half-lives of the medication. The determination of pain not significantly interfering with sleep was based on a rating of less than 25 mm on a 100-mm visual-analogue scale indicating pain interfering on a continuum between “not at all” and “extremely.” When eligibility preceded discharge, recordings were conducted from patients’ hospital beds (N=10) by using a portable polysomnographic recording unit. The remaining 11 patients returned to the hospital setting and were recorded from a sleep laboratory bed within 2 to 14 days of discharge. The mean number of days from the traumatic incidents to the polysomnographic recording were similar for the groups recorded during their admissions (mean=16.3, SD=10.1) or after discharge (mean=18.2, SD=7.6). While the goal was to obtain two consecutive recordings, timing of narcotic discontinuation and discharge and injury-related constraints on transportation limited this to accomplishment with only nine of the injured subjects. In order to maintain consistency between groups, primary analyses were conducted on first-night recordings. In the subgroup of injured patients with two nights recorded, mean values for sleep latency similar to those from the first night (mean=12.9 minutes, SD=16.1) and the second night (mean=12.1 minutes, SD=15.6) and sleep duration (total sleep time) (night 1 mean=297.9 minutes, SD=82.7; night 2 mean=306.5 minutes, SD=78.4) suggested stable habituation to the sleep environment. In an attempt to create somewhat homologous conditions, most of the noninjured comparison subjects (eight of 10) had unrecorded overnight habituation to the sleep environment before polysomnographic recordings. Recordings for all subjects used two standard central and reference EEG leads, bilateral horizontal electro-oculograms, submental electromyogram, an ECG lead, finger oximetry, and respiratory strain gauge monitors.
Each 30-second epoch of the sleep recording was visually scored from a computer screen by using standard criteria
(27). The four technicians who scored patients for the study had all been trained and achieved the threshold of 90% concordance for epoch staging with a criterion record. Epoch stages were entered into a computer program that calculated standard measures of sleep initiation, maintenance, and architecture. Sleep latency was defined as the time from “lights out” to the first epoch of at least 10 minutes of uninterrupted sleep. The amount of wake activity after sleep onset before the final awakening (wake during sleep) was used for the index of sleep maintenance. REMs occurring during REM sleep were identified by a previously used criterion of excursions of at least 25 μV occurring within 200 msec
(28). All scorers for the study achieved correlations of r>0.90, with a criterion tabulation of the number of REMs. REM density was calculated by dividing the number of REMs by minutes of REM sleep. This calculation was made for the entire night as well as for the first period of REM sleep lasting at least 3 minutes, as both initial REM density and total REM density have been considered indices of REM “pressure”
(29). REM sleep periods were determined by calculating the time from the beginning of at least two consecutive epochs of REM sleep to the last epoch of any subsequent REM sleep not separated by more than 20 minutes of non-REM sleep. Since a conventionally defined REM period can include substantial amounts of non-REM sleep, we additionally determined the duration of continuous REM sleep by calculating the time from the onset of at least 1 minute of REM sleep to the occurrence of at least two consecutive epochs (1 minute) of non-REM sleep or wake time.
Exploratory analyses were conducted to determine if there were relationships between sleep measures and location of the polysomnographic recording (inpatient bed or sleep laboratory) (grouped t tests) or time from the traumatic incident (Pearson’s product-moment correlations). Sleep measures, including the average duration of continuous REM sleep for subjects, were then compared among the injured patients who were positive and negative for PTSD symptoms at follow-up and the noninjured healthy comparison subjects by analysis of variance with post hoc testing with least-square difference set at p<0.05. Relationships of initial (concurrent) and follow-up PTSD severity and sleep measures in the combined group of injured patients were evaluated by calculation of Pearson’s correlations.
Results
Diagnosis
At the time of the follow-up evaluation, six of the 21 injured subjects met full criteria for PTSD. An additional four subjects met criteria for two of the three DSM-IV diagnostic clusters (all were one or two symptoms shy of the cluster C numbing and avoidance criteria) and were experiencing distress from their psychiatric symptoms. These 10 patients were grouped as positive for PTSD symptoms. This grouping provided a more balanced statistical comparison but also appears to be more effective than including only patients with full criteria for delineating subjects with and without significant trauma-related psychiatric morbidity. In an epidemiological sample, “partial” PTSD was found to be associated with levels of impairment in home and social functioning and in rates for seeking help similar to those with full PTSD
(30). The mean 17-item Clinician-Administered PTSD Scale score at follow-up for our group with PTSD was 49.6 (SD=14.7), and the mean score for the injured group without significant PTSD symptoms was 13.2 (SD=9.9) (t=6.3, df=19, p<0.001). Two of the 10 patients with PTSD met criteria for major depressive disorder that had developed between the time of their polysomnographic recording and the follow-up assessment and was determined to be secondary in onset and severity to PTSD.
None of the subjects’ polysomnographic recording findings indicated the presence of a sleep-related breathing disorder.
Sleep Measures and PTSD
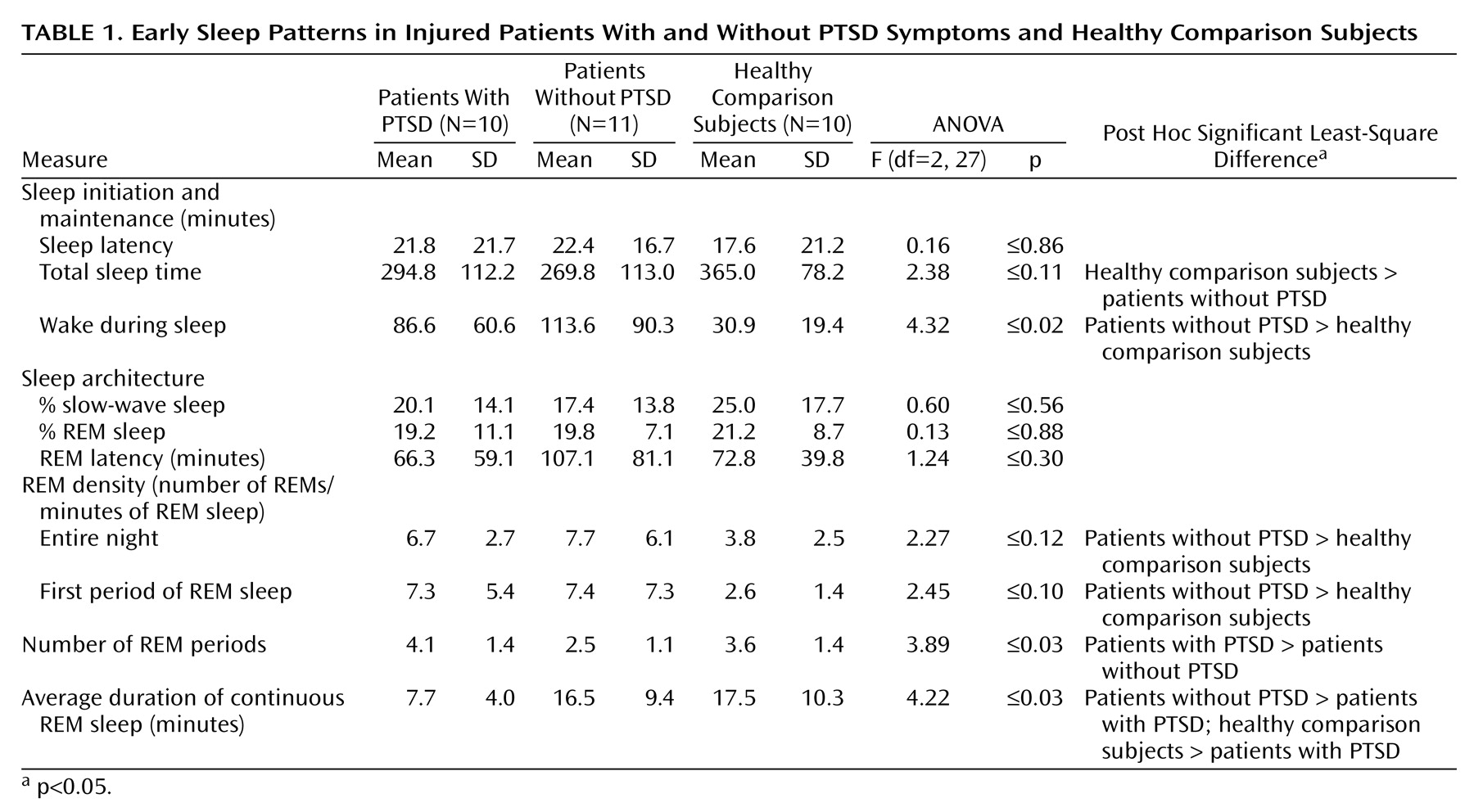
Comparisons of sleep measures among the injured patients with and without PTSD subjects and the noninjured comparison subjects are shown in
Table 1. Analyses reveal a significant difference for wake during sleep, with post hoc testing indicating more wake during sleep in the group without PTSD than in the comparison subjects. There was a main effect for initial REM density, with significantly greater activity in the group without PTSD than in the noninjured comparison subjects by post hoc testing. Significant main effects were found for the number of REM periods and the average duration of continuous REM sleep. The group members with PTSD exhibited a greater number of REM sleep periods than the group without PTSD; they also had a shorter average duration of continuous REM sleep than the group without PTSD and the noninjured comparison subjects. Mean sleep measures were similar for injured subjects recorded in the sleep laboratory versus in their hospital beds; none of the comparisons achieved significance. None of the correlations between sleep measures and time from the traumatic incident to the polysomnographic recording achieved significance except for the average duration of continuous REM sleep (r=–0.50, p<0.04). The difference for this measure between patients with and without PTSD remained significant when time from the incident was incorporated into an analysis of covariance (F=7.71, df=1, 19, p<0.01).
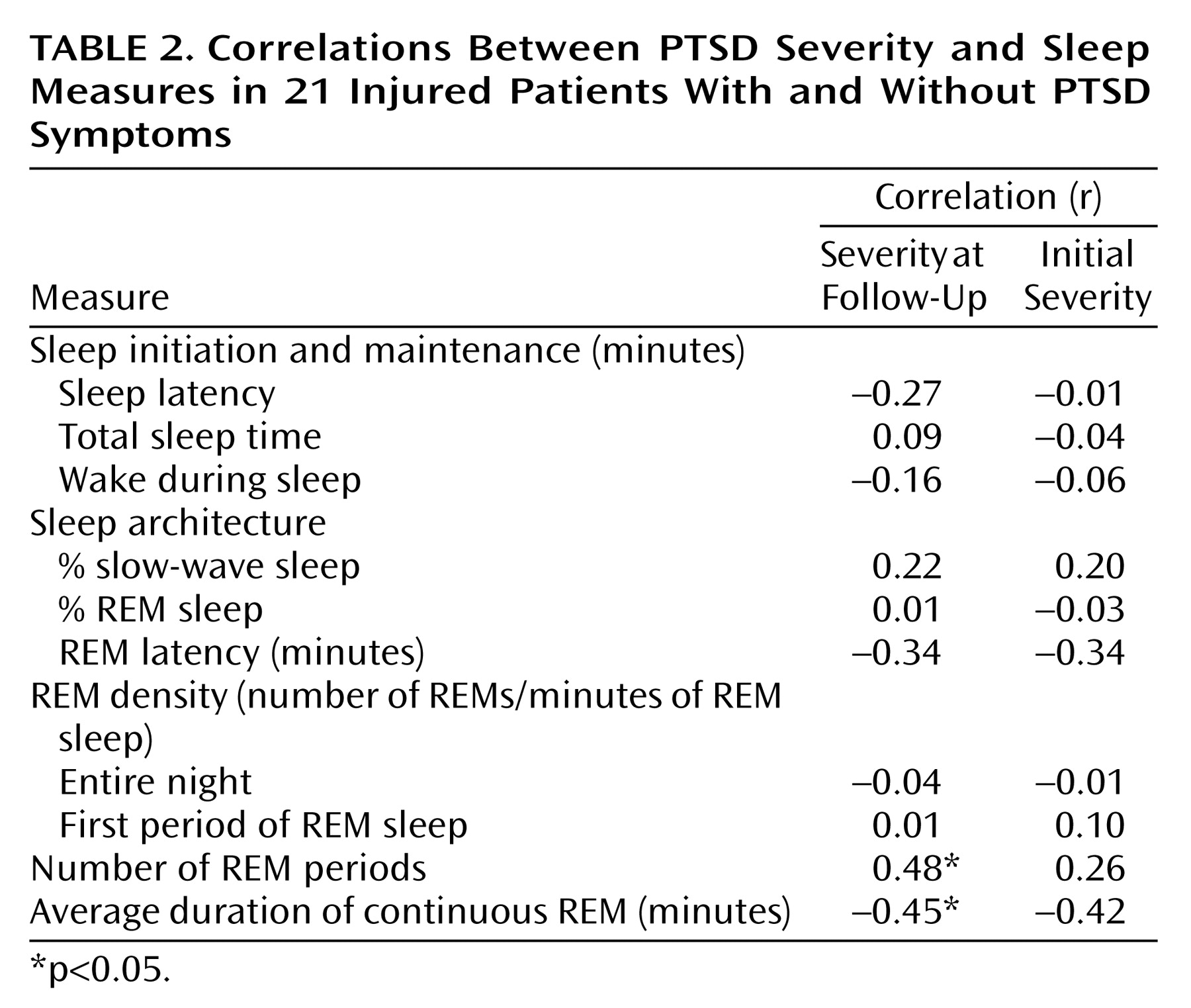
Correlations between PTSD severity and sleep measures for the combined injured patients with and without PTSD are listed in
Table 2 and demonstrate similar relationships. The correlations between PTSD severity and the indices of sleep maintenance, architecture, and REM density were weak and not significant. Follow-up PTSD severity was significantly positively correlated with the number of REM sleep periods and negatively correlated with the average duration of continuous REM sleep. Relationships were similar but not as consistent for these sleep measures and initial (concurrent) PTSD severity.
Discussion
This study provides unique data, albeit with limitations, on relationships of polysomnographic sleep measures within a month of life-threatening trauma and injury and subsequent PTSD. It is not surprising that there was evidence for disrupted sleep in the injured patients relative to the healthy comparison subjects. Sleep duration and maintenance were similar between the injured groups with and without PTSD symptoms at follow-up, however. The group that developed PTSD symptoms was distinguished by findings indicating a more fragmented pattern of REM sleep. Specifically, they demonstrated shorter average duration of REM sleep before shifting stage or awakening than the group without PTSD and the noninjured comparison group, and a greater number of REM sleep periods than the group without PTSD.
Greater REM density has been noted with chronic PTSD
(16,
18). Our findings were not supportive of a relationship of eye-movement density to PTSD during an acute posttrauma period but suggested a relationship to trauma exposure. Otherwise, our findings were not inconsistent with the studies we reviewed of chronic PTSD that are mixed with regard to evidence for impaired sleep maintenance and do not suggest characteristic alterations in the latency to, or amount of, REM sleep. There is some evidence suggesting disruption of REM sleep with PTSD
(20,
21) and acute combat fatigue
(22); however, prior PTSD sleep studies have not typically included the indices of REM continuity reported here. Fragmented REM sleep patterns may not be entirely specific to the early development of PTSD. Shorter sleep cycles have been noted in a subgroup of depressed patients who exhibited greater REM pressure with normal patterns of adaptation to the sleep laboratory environment
(31). Short REM periods have been reported with alcoholism (which was excluded in our cases) during both early and late stages of abstinence
(32).
Confidence in these findings is tempered by several study limitations. The number of subjects is modest. Despite screening criteria intended to limit potential confounds, a number of factors could have influenced sleep patterns. However, we were not able to identify any systematic differences between injury patterns or other potential confounds between the patients with and without PTSD. Logistics of recordings of injured subjects not taking medications and within an acute time frame limited us from consistently achieving the sleep research standard of obtaining recordings on multiple nights. As we mentioned, indices of sleep initiation and maintenance were similar for the injured patients on consecutive nights when they were available. Inspection of the data among the nine injured subjects with readings on consecutive nights (five with PTSD) suggests that while the number of REM periods and the duration of continuous REM sleep are not highly stable measures, the findings do not appear to be attributable to first-night effects. For example, the average duration of continuous (uninterrupted) REM sleep was shorter on the second night than the first night in six of the nine injured subjects (four of five with PTSD). Findings were also of similar magnitude and significance when the mean values for available consecutive nights were used instead of only first-night measures. Depression was also an outcome in two of the patients with PTSD, although criteria were not yet met at the time of the polysomnographic recording. While the number of patients precludes a meaningful statistical analysis, these patients featured extremes of REM latency values (10 and 193 minutes), were at the upper end of the distribution for REM sleep percent (36%, 23%), and were at the lower end for eye-movement density for the initial REM period (2.4, 3.8).
Limitations notwithstanding, this is the first study of which we are aware to report polysomnographic findings from an acute phase of trauma response in more than a few subjects and to include longitudinal assessment of PTSD. While our findings need confirmation, potential implications of a more fragmented pattern of REM sleep and the development of PTSD are intriguing. REM sleep features patterns of neuromodulation and cortical activation that are distinct from wake and other sleep states. The bizarreness that is typical of dream reports preceded by REM sleep (i.e., juxtaposition of characters, situations and time frames that are not normally or logically associated) is considered to be a function of activation of the association cortex without engagement of executive frontal brain structures
(33). In an experiment in which subjects were administered a semantic priming test just after being awakened from REM sleep, the priming advantage was for weakly associated word pairs. This was in contrast to a finding of maximal priming with strongly associated word pairs when subjects were fully awake
(34). Thus, conditions of REM sleep appear to favor coactivation of memory networks that are not typically associated in waking cognition. Recent studies indicate that REM sleep activity has an effect on memory that endures after awakening
(12,
13). These neurocognitive attributes may serve to extend associative networks and thereby underlie the adaptive integration function postulated for REM sleep in the literature
(9–
11). Incorporation of new associations in trauma memory is thought to be an important component of successful emotional processing during exposure therapy for PTSD
(35). Impairment in extending associations in relation to dreams is suggested by our previously reported findings from an overlapping population relating PTSD to dream content similar to trauma memory, in contrast to dreams that were dissimilar but often incorporated a trauma reference
(36).
The current study data suggests that adaptive functions of REM sleep after trauma may depend on its duration without disruption. While it is attractive to postulate disrupted REM sleep having a causal influence on developing PTSD, it is worth noting that central noradrenergic activation is linked to fear and anxiety states
(37) and termination of REM sleep
(38). This possible pathway does not preclude, however, a relationship between ensuing REM disruption and nonresolution of PTSD symptoms. (Our study’s correlations were stronger and more consistent for prospective than concurrent relationships between sleep and PTSD severity.) We hope that attempts to replicate and extend these findings will further clarify the relationships of sleep, the specific role of REM sleep, and the development of PTSD and point toward intervention strategies.