Patients suffering from borderline personality disorder constitute a substantial proportion of the consumers of mental health care and exhibit a broad spectrum of symptoms and behaviors: affect lability with rapid mood shifts from normal to depressive states, distinct irritability or anxiety, and impulsive, aggressive, and parasuicidal behavior. A considerable number of studies
(1–
3) point to the central serotonergic system as a possible target for psychopharmacological intervention for impulsive, aggressive, and suicidal patients and thus for patients with borderline personality disorder. Several open studies
(4–
9) of selective serotonin reuptake inhibitors (SSRIs) for small groups of borderline patients suggest that the prescription of an SSRI may be an effective pharmacological strategy to ameliorate the pathology of borderline personality disorder. However, we know of only one double-blind, placebo-controlled, randomized trial
(10), which involved 14 female and eight male borderline patients with mild to moderate symptoms, and the results did not confirm the positive results of the open studies because of a high rate of response to placebo. After post hoc refinement of the analysis and the introduction of a measure of placebo response, statistically significant differences were obtained with respect to anger and aggression. A statistically significant improvement in impulsive aggression was observed after 12 weeks in another double-blind, randomized SSRI study
(11), which included 28 male and 12 female subjects suffering from a variety of impulsive DSM-III-R personality disorders (13 subjects had borderline personality disorder). However, the authors warned that the results of this study must be interpreted with caution because of the substantial dropout rate (43%).
Beyond these promising but controversial results with SSRIs, we know of no further double-blind, placebo-controlled studies using larger numbers of borderline patients with severe borderline pathology. Moreover, on the basis of these findings from SSRI studies, SSRI treatment is recommended for borderline personality disorder patients with affect lability, impulsivity, and aggressiveness
(12,
13), a strategy also adopted in the practice guideline on borderline personality disorder of the American Psychiatric Association
(14).
Patients with borderline personality disorder are susceptible to a broad spectrum of axis I disorders, such as affective disorders, anxiety disorders (including posttraumatic stress disorder [PTSD]), eating disorders, and substance use disorders
(15). In all of the SSRI treatment studies of personality disorder patients that we know of, current major depressive disorder was an exclusion criterion because of the antidepressive effects of SSRIs. Such a criterion, however, does not allow for the fact that SSRIs are also an effective treatment for many patients with anxiety disorders (including PTSD) and eating disorders
(16–
18). Furthermore, exclusion of all borderline patients with one or more coexisting axis I disorders would reduce the study group to a marginal group with mild pathology. A clinically more relevant research strategy might, therefore, be a less rigorous exclusion of comorbid axis I disorders and inclusion of them as a covariate in the statistical analysis.
In addition to the preceding complications, the severity of borderline pathology raises some major ethical and practical issues with regard to the use of long-term double-blind, placebo-controlled treatment. Taking this into account, we decided to design the study in such a manner that the length of the placebo-controlled phase would be kept to a minimum but without the loss of statistical power.
We believe that the present study includes the largest group (N=38) of well-diagnosed borderline personality disorder patients with moderate to severe pathology so far studied in a pharmacotherapy trial. We conducted a double-blind, placebo-controlled, randomized trial using the SSRI fluvoxamine for 6 weeks followed by a blind half-crossover (with fluvoxamine replacing the placebo) for 6 weeks and an open follow-up (in which all patients were offered fluvoxamine) for another 12 weeks. Following the mentioned treatment recommendation, we investigated the effects of fluvoxamine on the borderline symptoms of rapid mood shifts, impulsivity, and anger. Potential effects of interactions with relevant axis I disorders were taken into account in the statistical analysis.
Method
Subjects
Physically healthy women between the ages of 18 and 50 years were recruited from psychiatric outpatient clinics, from community mental health centers, and by advertising in newspapers and on the Internet. Given this heterogeneous recruitment method, a rigorous diagnostic procedure was applied to select a homogeneous study group with moderate to severe DSM-IV borderline personality disorder. In order to be included in the study, all patients had to 1) obtain a score of 110 or more on the borderline trait and distress scale of a self-report screener for personality disorders, the Assessment of DSM-IV Personality Disorder
(19), 2) meet five or more of the criteria on a semistructured diagnostic interview, the Structured Interview for DSM-IV Personality Disorders
(20,
21), and 3) receive a score of 20 or more on a fully structured interview, the Borderline Personality Disorder Severity Index
(22,
23). The screening instrument was returned by 125 referred or self-referred subjects. Of these, 78 had a score higher than 110 and were invited for further diagnostic interviews. The final study group comprised the 38 subjects eligible for participation; 21 were referred by outpatient clinics, and 17 volunteered for participation after reading a newspaper article about the study or visiting a web site on borderline personality disorder. Two of the 17 self-referred subjects had never received psychiatric treatment before, and three had discontinued treatment in the past because of dissatisfaction. The participants had to stop taking all psychoactive drugs after signing the informed consent statement, and they all had to be medication free for at least 2 weeks before entering the trial; the medication-free interval was 6 weeks for fluoxetine. All patients were given full information in both written and oral form and were asked to provide written consent before entering the study.
Design
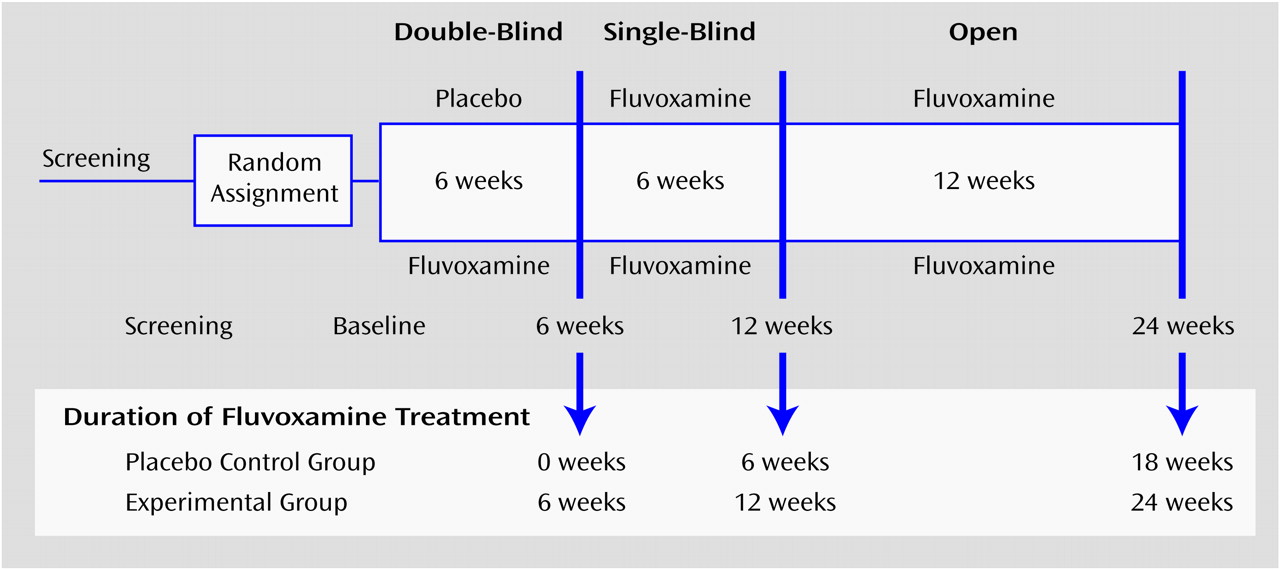
The study consisted of a double-blind, placebo-controlled, randomized trial of 6 weeks, followed by a half-crossover for another 6 weeks and an open treatment period of 12 weeks to evaluate the effects of fluvoxamine on borderline pathology (
Figure 1). The 38 subjects were randomly allocated to the fluvoxamine and placebo conditions. After randomization, the subjects entered the double-blind, placebo-controlled phase of 6 weeks. At the end of this period, all subjects received fluvoxamine for 6 weeks without the breaking of the randomization code (half-crossover). In cases of insufficient clinical improvement according to the treating physician after week 10, an adjustment of the fixed daily dose of 150 mg of fluvoxamine could be made (to a maximum of 250 mg). The 6-week single-blind phase was followed by a 12-week open phase with fluvoxamine prescribed to all subjects. In week 10, the dose of five subjects was adjusted to 200 mg/day (mean=155.71, SD=20.18); in week 12, 11 subjects used a dose of 200 mg/day (mean=165.62, SD=26.75); and in week 24 also, 11 subjects used 200 mg/day (mean=167.24, SD=27.63).
Psychiatric assessments took place at screening, at baseline just before randomization, and at weeks 6, 12, and 24. Adverse events were recorded every 2 weeks.
Diagnoses
Borderline personality disorder
Prescreening for the presence of DSM-IV borderline personality disorder was performed with a self-report questionnaire, the Assessment of DSM-IV Personality Disorder
(19). This questionnaire consists of 94 items representing the 80 criteria for the 10 different DSM-IV personality disorders. The item response format emphasizes the pathology conceptualization of DSM-IV by scoring an item on both a 7-point scale measuring the trait (1=absolutely disagree, 7=absolutely agree) and a 3-point scale measuring trait-related distress (1=absolutely no distress, 3=definite distress). If a subject scores 5 or higher on the trait scale, he or she also has to indicate the degree of distress on the 3-point distress scale. Computation of the answers follows a scoring algorithm, which provides a total score for each personality disorder on a combined trait and distress scale. In order to move to the next phase in the diagnostic procedure, each subject had to score at least 110 points on the borderline personality disorder subscale of the Assessment of DSM-IV Personality Disorder.
The presence or absence of DSM-IV borderline personality disorder was established with the Dutch version of the Structured Interview for DSM-IV Personality Disorders
(20,
21). Three psychologists, who were specially trained by the Dutch translators of the instrument, administered the interview. The interviews were videotaped and reassessed by another interviewer, who was blind to the diagnosis. The final diagnosis was obtained by consensus. In order to be included in the study, each subject had to meet at least five of the nine criteria for borderline personality disorder.
The Borderline Personality Disorder Severity Index
(22,
23) was used to select only the patients with moderate to severe borderline pathology. The index is a fully structured interview that measures the frequency of occurrence of all DSM-IV borderline personality disorder criteria over the past 3 months. Each of the nine DSM criteria is operationalized as a subscale, consisting of three to 11 items. The frequency of each item is measured on an 11-point scale on which 0 indicates no occurrence, 1 represents one occurrence in 3 months, 5 represents six or seven occurrences in 3 months or once every 2 weeks, and 10 indicates daily occurrence. The mean score on the items constitutes the subscale score, and the sum of the subscale scores is the total score. The cutoff for the Borderline Personality Disorder Severity Index is a total score of 15. In order to be included in the study, subjects in this study had to score 20 or more on the Borderline Personality Disorder Severity Index (the mean score in this study was 32.9, SD=7.7).
Axis I disorders
The Composite International Diagnostic Interview, lifetime module
(24,
25), is a fully structured interview aimed at the diagnosis of DSM-IV and ICD-10 disorders, including schizophrenia, substance use disorders, unipolar and bipolar affective disorders, anxiety disorders (including PTSD), and eating disorders. All researchers were trained by the staff of the Dutch World Health Organization Composite International Diagnostic Interview Training and Reference Center in Amsterdam.
Outcome Measures
To test our hypothesis, three subscales of the Borderline Personality Disorder Severity Index were selected. The subscale for rapid mood shifts contains three questions concerning brief mood shifts (i.e., changes from a normal mood to depression, irritability, and/or anxiety). The anger subscale contains four questions concerning verbal and physical aggression as well as fits of temper. The impulsivity subscale contains 11 questions concerning several (potentially) harmful and therefore pathologically impulsive behaviors: buying things impulsively, unsafe sex, sex with unknown persons, gambling, excessive use of alcohol, excessive use of soft drugs (such as marijuana or hashish), use of hard drugs, binge eating, shoplifting, reckless driving, and other potentially dangerous impulsive behaviors (excluding parasuicidal behavior).
In two substudies among patients with different personality disorders and healthy comparison subjects, the Borderline Personality Disorder Severity Index and its subscales were shown to have excellent interrater reliability, as indicated by the intraclass correlation coefficient (impulsivity: 0.82–0.99, anger: 0.71–0.93, rapid mood shifts: 0.89–0.97); acceptable internal consistency, as indicated by Cronbach’s alpha (impulsivity: 0.67, anger: 0.69, rapid mood shifts: 0.80); and good concurrent and discriminant validity
(22). In addition, it was shown that the 3-month version of the Borderline Personality Disorder Severity Index is sensitive to change
(22). To evaluate the symptoms at 6-week intervals, the 3-month time frame associated with the original instrument was changed into a 6-week time frame so that it would be suitable for assessment at 6, 12, and 24 weeks after randomization. In the current study, the values for internal consistency (Cronbach’s alpha) of the Borderline Personality Disorder Severity Index subscales at 12-week follow-up were 0.48 for impulsivity, 0.69 for anger, and 0.82 for rapid mood shifts.
Statistical Analyses
To test the efficacy of fluvoxamine after the 6-week double-blind, placebo-controlled phase of the study, an intent-to-treat analysis was performed by using the scores from the Borderline Personality Disorder Severity Index subscales as the primary outcome variables. Separate analyses of covariance (ANCOVAs) were performed with the 6-week score on each of the subscales as the dependent variable, the baseline score on the subscale as a covariate, and treatment group (fluvoxamine or placebo) as the independent variable. Dropouts were taken into account in the intent-to-treat analyses by using an unbalanced repeated measure model with structured covariance matrices
(26).
To test the probable influence of coexisting axis I disorders on the improvement of borderline personality disorder symptoms with fluvoxamine treatment, a series of two-by-two ANCOVAs were performed by using the week 6 rating for each Borderline Personality Disorder Severity Index subscale as the dependent variable, the baseline subscale score as a covariate, treatment group and the presence of a comorbid diagnosis of depression or PTSD as independent variables, and an interaction term for treatment group and the presence of a comorbid axis I disorder. A significant interaction term (representing the interaction of treatment and comorbid axis I disorder) was taken to indicate significant mediation of the fluvoxamine treatment effect by the presence of comorbid axis I disorders.
To explore the speed of recovery, we examined the total scores on the Borderline Personality Disorder Severity Index subscales for the experimental and initial placebo groups at multiple time points. Endpoint scores were obtained at week 24, after 24 weeks of fluvoxamine treatment for the experimental group and after 18 weeks of fluvoxamine treatment for the initial placebo group (
Figure 1). Thus, we examined the total scores of the experimental group and initial placebo group at baseline before they began fluvoxamine treatment (experimental group at week 0, N=20; placebo group at week 6, N=18), for both groups after 6 weeks of fluvoxamine treatment (experimental group, N=19; placebo group, N=18), for the experimental group after 12 weeks of fluvoxamine treatment (week 12) (N=17), for the original placebo group after 18 weeks of fluvoxamine treatment (week 24) (N=14), and for the experimental group after 24 weeks of fluvoxamine treatment (week 24) (N=16). Two types of analyses were then performed: 1) analyses using the existing data from partly different study groups at the different points in time (real data matrix) and 2) analyses using the covariance matrix for nonmissing values as the basis for imputation of missing values, resulting in estimates for all 38 subjects at all points in time (completed data matrix)
(26). Three separate multivariate analyses of variance (MANOVAs) with repeated measures were conducted on the completed data matrix with the three Borderline Personality Disorder Severity Index subscales as dependent variables. Helmert contrasts were used to evaluate significant changes.
All of the statistical tests were conservatively two-tailed and used a 5% level of significance.
Results
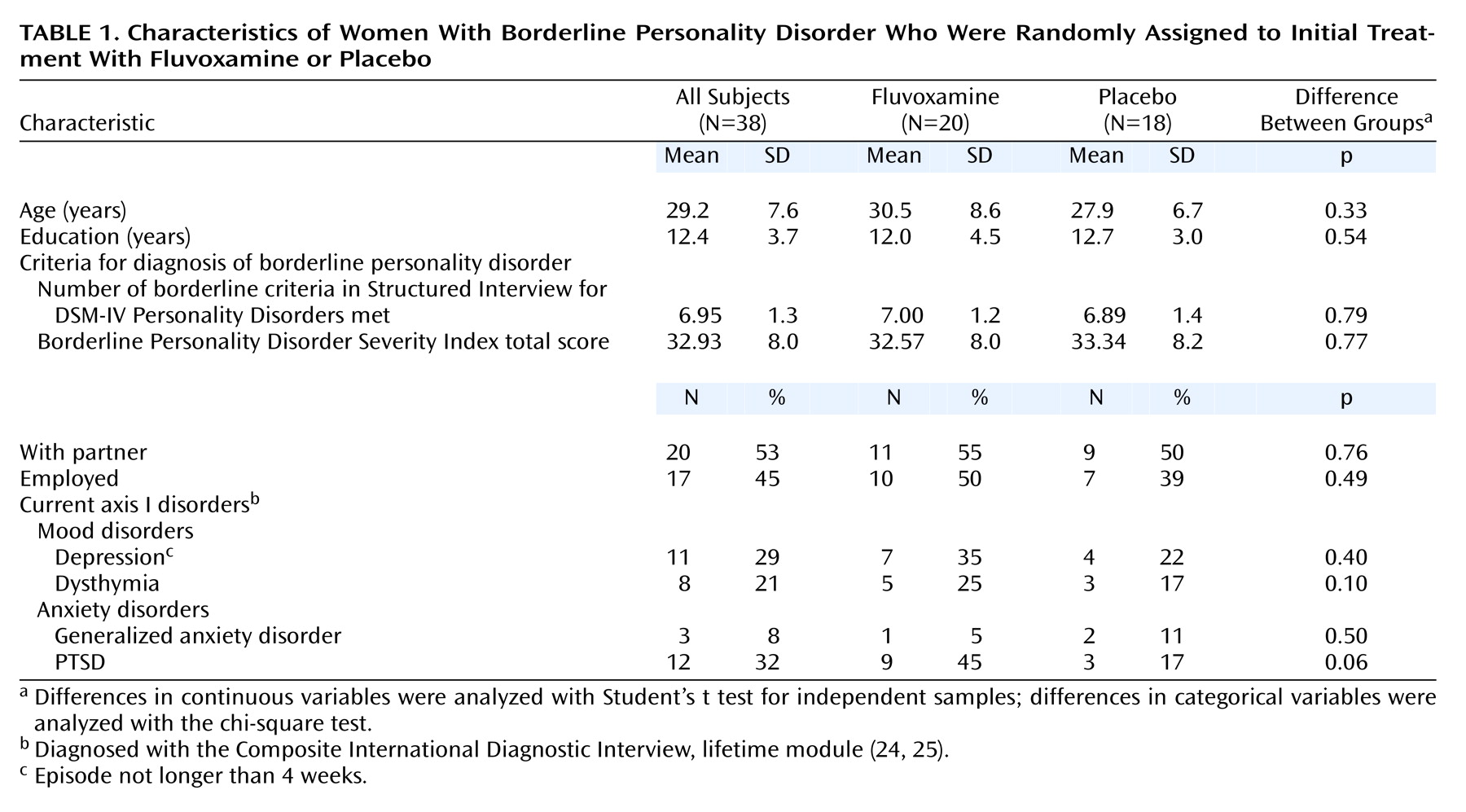
Table 1 shows the randomization process to have been successful. No significant differences between the fluvoxamine and placebo group in terms of sociodemographic variables, axis I disorders, or personality traits were observed at baseline. It should be noted, however, that the difference in the prevalence of PTSD between the two groups almost reached statistical significance (45% versus 17%) (p=0.06).
Effect of Fluvoxamine on Borderline Symptoms
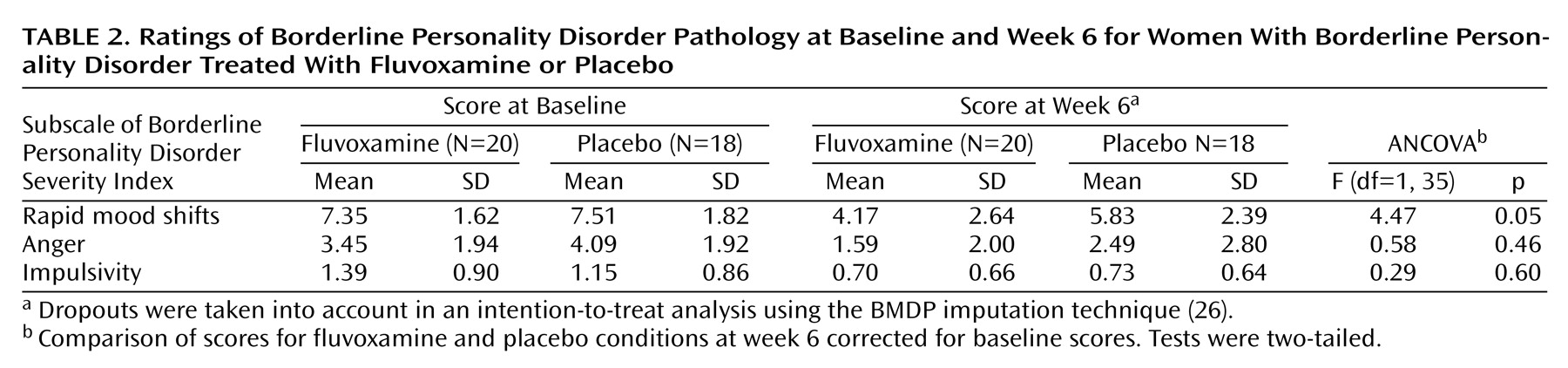
Table 2 shows the effects of fluvoxamine on borderline personality disorder pathology. Although significant decreases on all three subscales of the Borderline Personality Disorder Severity Index were observed in both conditions after 6 weeks, a larger decrease for the fluvoxamine group than the placebo group was observed only for the rapid mood shifts subscale. The results of a two-by-two ANCOVA indicated that the effects of fluvoxamine on the subscale scores are likely to occur independent of comorbid affective disorders or PTSD.
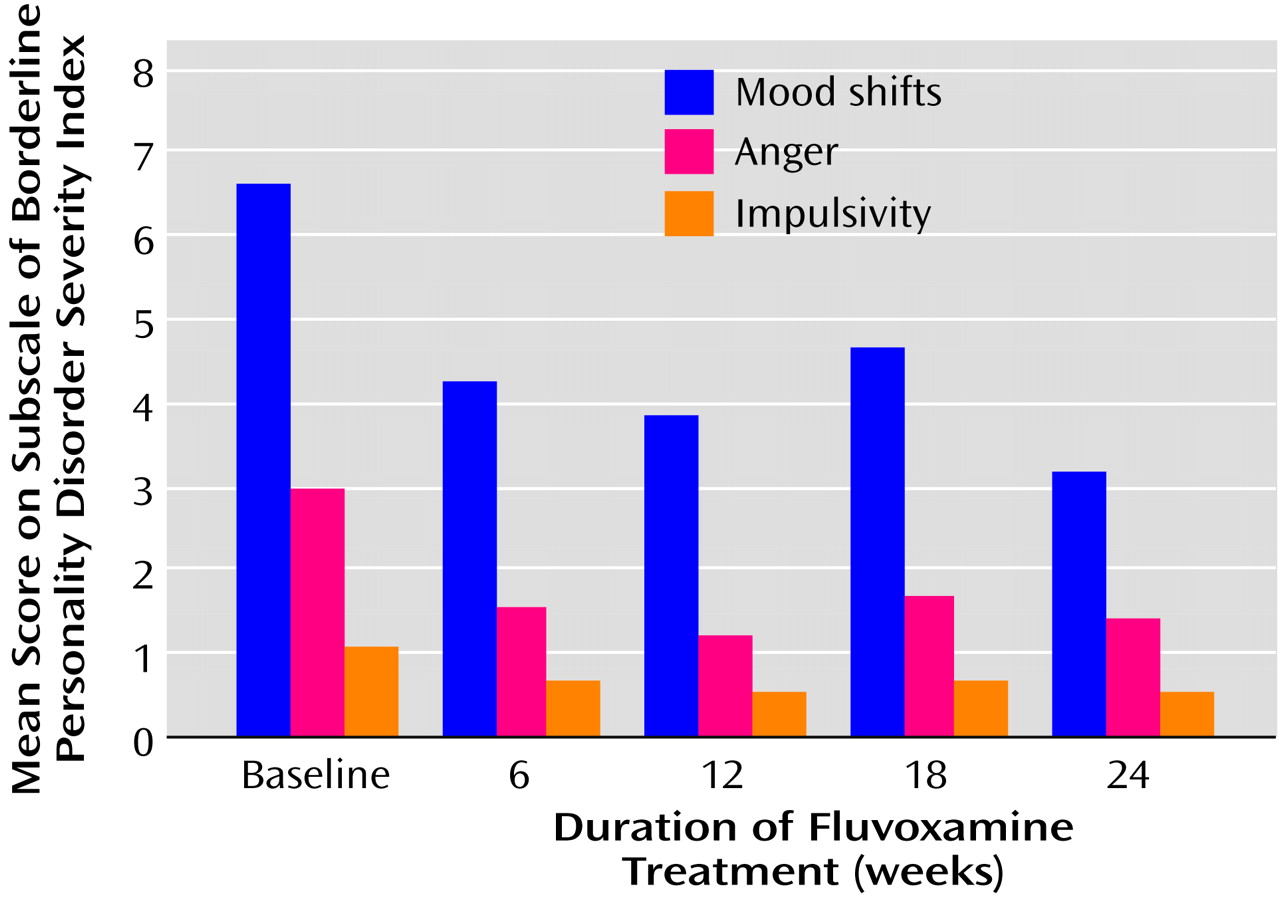
Figure 2 shows that the main effect of fluvoxamine is in the first 6 weeks. A repeated measures analysis of variance revealed a significant reduction between baseline and week 24 in the scores on the three Borderline Personality Disorder Severity Index subscales. Post hoc Helmert contrast analysis also showed that the most substantial symptom decrease took place during the first 6 weeks of treatment with fluvoxamine. There was no significant difference between the scores after 6 weeks of treatment and those after 12, 18, or 24 weeks of treatment.
Adverse Events and Dropouts
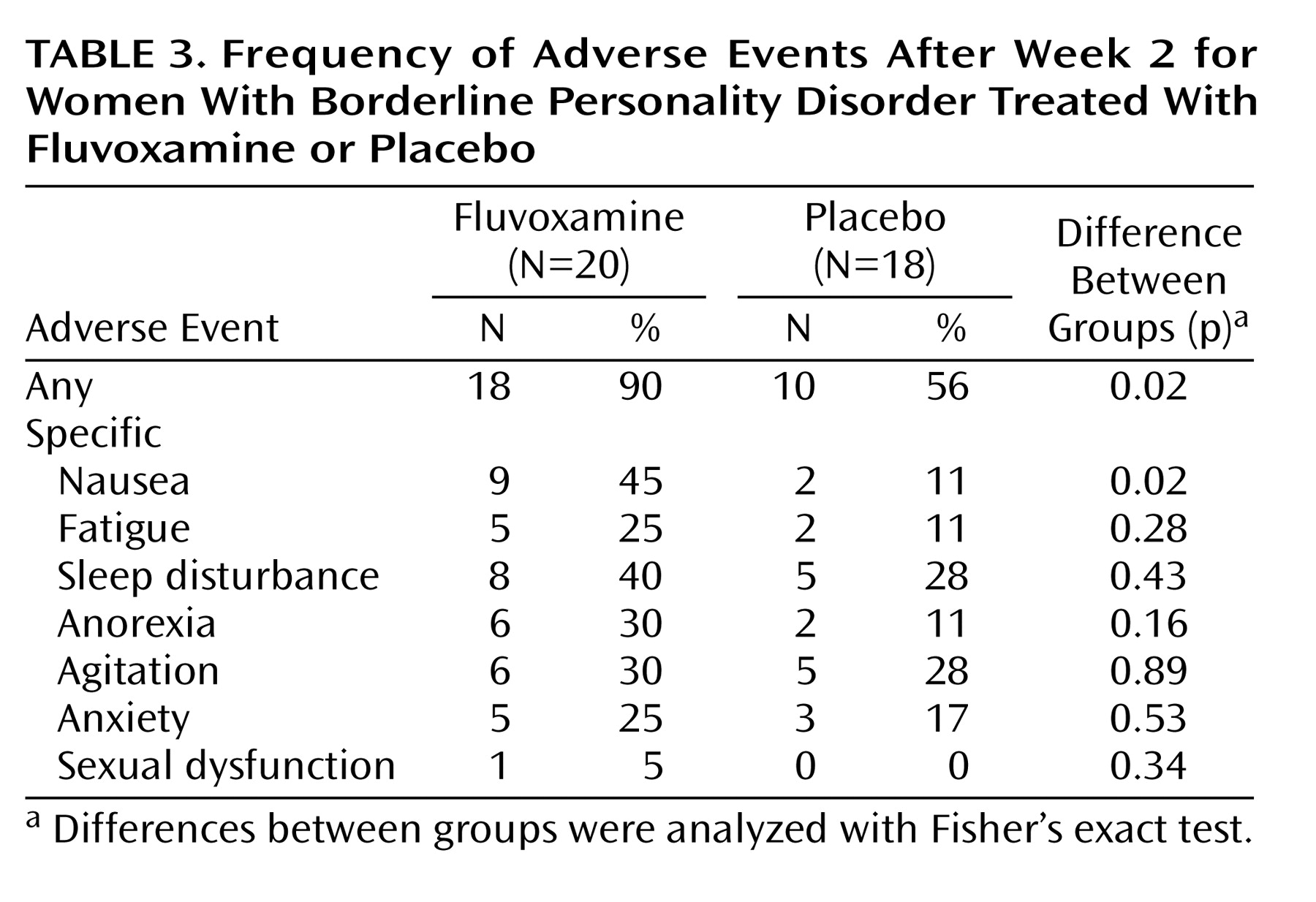
Table 3 shows that many of the patients in the placebo group as well as in the fluvoxamine group reported adverse events after the initial 2 weeks of the study. In general, the fluvoxamine group had significantly more complaints than the placebo group, although the only specific problem for which there was a significant difference was nausea.
During the placebo-controlled phase, two subjects in the placebo condition dropped out because of serious aggravation of self-damaging behaviors, and they were treated openly with fluvoxamine after symptom assessment. In addition, one subject in the fluvoxamine group dropped out because of severe side effects; thus, the dropout rate for the experimental group was 5% (one of 20). During the 24 weeks of the study, a total of nine subjects (24% of the total group) dropped out: two because of lack of efficacy, two because of adverse events, two who began psychotherapy, and three who did not provide comments.
Discussion
This randomized, double-blind, placebo-controlled SSRI trial for 38 women with borderline personality disorder of moderate severity demonstrates a statistically significant and clinically relevant effect of fluvoxamine on the occurrence of rapid mood shifts. The effect was found to occur independent of coexisting PTSD or depression. Moreover, this effect is reached within the first 6 weeks of treatment and seems to persist during the follow-up of 18 weeks. No effect of treatment with fluvoxamine could be observed for the other two subscales of the Borderline Personality Disorder Severity Index, measuring pathological anger and impulsivity, because the reductions in the placebo and fluvoxamine groups were similar. This finding corroborates the results of the only other double-blind, placebo-controlled SSRI study with borderline personality disorder patients of which we are aware
(10).
A question that remains to be answered is whether particular limitations of the current trial may account for the lack of an effect of treatment with fluvoxamine on anger and impulsivity. First, the dose of fluvoxamine, 150 mg/day, may have been too low for the treatment of impulsive and aggressive behavior. However, this dose was chosen because it is fairly high and is sufficient for most indications but is low enough to restrict side effects. Fluvoxamine inhibits its own metabolism; an increase of the daily dose from 150 to 200 mg can increase the fluvoxamine plasma level by 100% and considerably increase the adverse effects of the medication and, as a result, the dropout rate. The fact that from week 12 to week 24 one-third of the remaining subjects used a dose of 200 mg/day could be an indication of a physiological adjustment to the drug and the need of a higher dose to maintain the symptom improvement. However, the increased doses had no further statistical impact on the measurements at weeks 12 and 24. Second, it cannot be ruled out completely that the power of the study was too small to detect smaller differences in reductions of anger and impulsivity and interactions with depression. Furthermore, the failure to detect an improvement in impulsive and aggressive behavior after fluvoxamine treatment might be related to the fact that only female patients without alcohol and drug problems were included in the study. Finally, the lack of an effect of fluvoxamine on impulsivity might also be related to the relatively low internal consistency of the impulsivity subscale of the Borderline Personality Disorder Severity Index (alpha=0.48).
Until now, we believe that the correlation between impulsive aggressive behavior and serotonergic hypofunction has been observed exclusively in Caucasian men (see reference
27 for review) and has not been demonstrated in women
(28–
30). Furthermore, in a serotonergic challenge study with
m-CPP, we found no change in the severely blunted prolactin and cortisol responses to
m-CPP after 8 weeks of open treatment with fluvoxamine for 10 female patients with borderline personality disorder (unpublished report). Moreover, the blunting of the prolactin response to
m-CPP was highly correlated with sustained childhood abuse but not with impulsive and aggressive behavior
(30). These data challenge the serotonin hypothesis with respect to impulsive aggressive behavior in women.
In sum, the present study addresses a major problem for evidence-based medicine and the pharmacological treatment of borderline patients. SSRIs are generally regarded to be effective for the treatment of the affective dysregulation and impulsive aggression found in borderline patients
(12,
13). The findings of at least 10 open SSRI studies all show statistically significant improvements, and extrapolation from other studies of the effects of SSRIs on impulsive aggression among patients with other personality disorders provides additional support for this assumption
(9). However, the only randomized, double-blind, placebo-controlled study with exclusively borderline personality disorder subjects that we could locate did not replicate the findings on impulsivity and aggression of the open SSRI studies and the study with different impulsive personality disorders. Our study provides at least some support for SSRI treatment of rapid mood shifts in female borderline patients. The scientific discussion of the role of SSRIs in the treatment of borderline pathology is therefore far from closed.
Additional randomized, double-blind, placebo-controlled SSRI studies are needed and should involve larger numbers of both female and male patients with borderline personality disorder. In addition, more sophisticated research strategies must be developed to solve the problem of high rates of response to placebo by patients with borderline personality disorder and high dropout rates of especially impulsive subjects. Therefore, the duration of the placebo control phase should be restricted to a maximum of 6–12 weeks. Finally, research guidelines should be developed to establish the use of a standard set of outcome measures in order to facilitate comparisons across studies.