A total of 167 subjects were randomly assigned to a treatment group, but 10 of them terminated before receiving study medication. Thus, the study is based on data from 157 subjects randomly assigned to treatment with clozapine (N=40), olanzapine (N=39), risperidone (N=41), or haloperidol (N=37). Their diagnosis was schizophrenia (86.0%, N=135) or schizoaffective disorder (14.0%, N=22). There were 133 male subjects (84.7%). The mean age was 40.8 years (SD=9.2), mean duration of illness was 19.5 years (SD=8.4), and the mean number of hospitalizations was 10.5 (SD=8.3). There were no statistically significant differences among treatment arms on any demographic variable. After complete description of the study to the subjects, written informed consent was obtained.
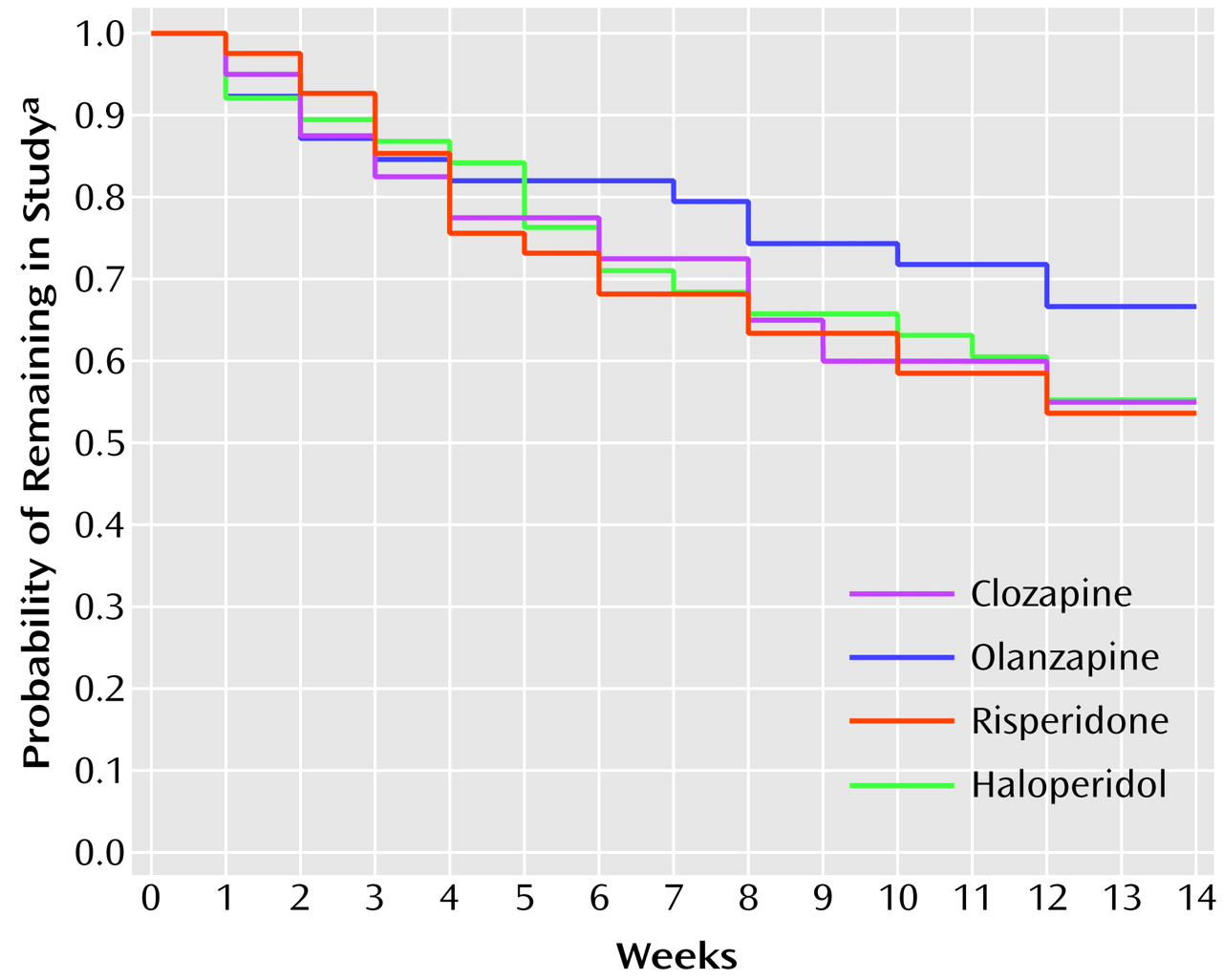
The 14-week study was completed by 91 (58.0%) of the 157 subjects. The Kaplan-Meier estimates of the probability of completing the study are displayed in
Figure 1. The differences in the attrition rates among treatments were not statistically significant (log rank test: χ
2=1.52, df=3, p=0.68). The most frequent reason for premature discontinuation (N=22) was consent withdrawal (five patients receiving clozapine, four receiving olanzapine, eight receiving risperidone, and five receiving haloperidol). Clinical deterioration caused premature termination in 14 patients (two receiving clozapine, four receiving olanzapine, two receiving risperidone, and six receiving haloperidol). Six patients were discharged and could not be followed up (three receiving risperidone, and one each from the other three treatment arms). Hematological problems led to discontinuation in three patients receiving clozapine; seizures caused premature termination in four patients (two receiving clozapine, two receiving risperidone). The remaining 17 premature discontinuations occurred for administrative reasons (one receiving clozapine, two receiving olanzapine, four receiving risperidone, and three receiving haloperidol), intercurrent illnesses (three receiving clozapine, one receiving olanzapine, and one receiving haloperidol), or protocol violations (one receiving clozapine, one receiving olanzapine).
Antipsychotic Efficacy
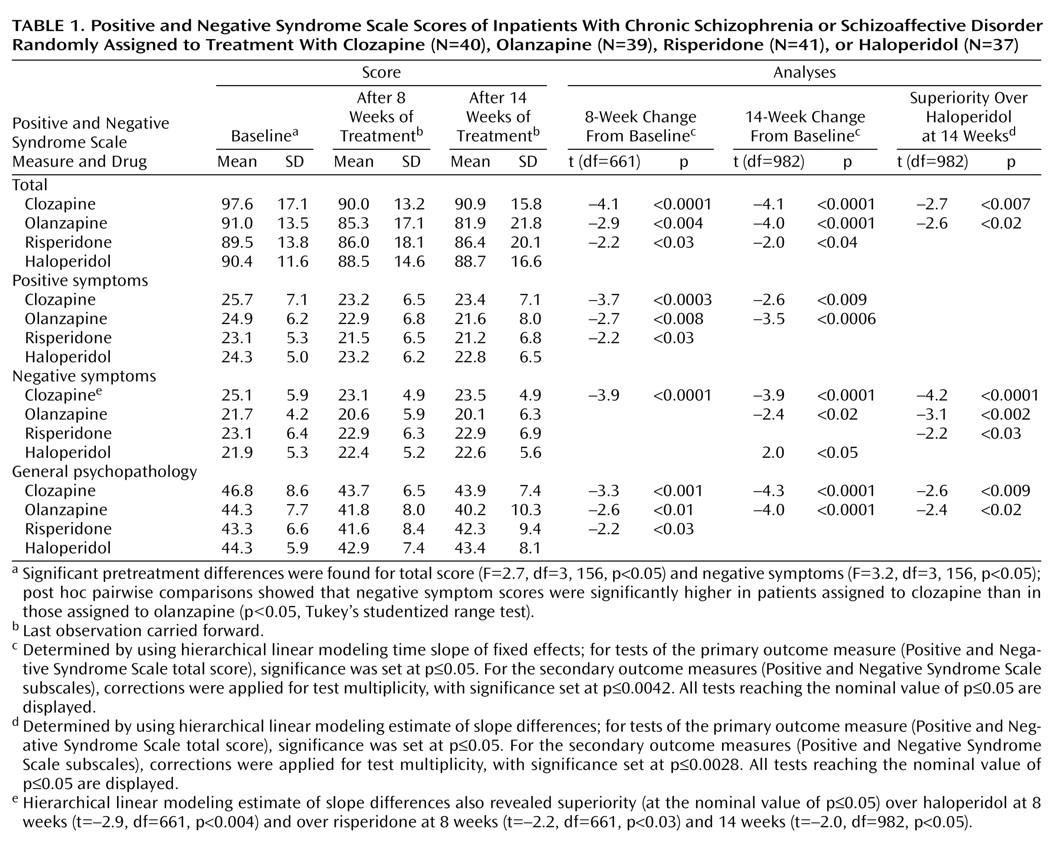
The Positive and Negative Syndrome Scale mean scores and standard deviations of the last observations carried forward are in
Table 1 (data for each time point are available on request).
Analyses of variance showed statistically significant differences among the treatment groups at baseline for the total scores and for negative symptoms. The total scores at baseline were higher in the clozapine group than in the other groups, but post hoc pairwise comparisons (six separate Tukey studentized range tests comparing treatment groups with each other) indicated no statistically significant differences between treatment groups for the total score. However, post hoc pairwise comparisons showed that baseline negative symptom scores in the clozapine group were significantly higher than in the olanzapine group (
Table 1).
The hierarchical linear modeling analysis of the data for the 14-week trial indicated a statistically significant change over time for each of the Positive and Negative Syndrome Scale measures (total score: F=23.9, df=1, 153, p<0.0001; positive symptoms: F=21.6, df=1, 153, p<0.0001; negative symptoms: F=6.3, df=1, 153, p<0.02; general psychopathology: F=23.7, df=1, 153, p<0.0001). The time effects were similar after the first 8 weeks of the trial (available on request).
Hierarchical linear modeling tests for fixed effects were used as the first step in the direct comparison of treatments. For the primary measure of efficacy (total score on the Positive and Negative Syndrome Scale for the entire 14-week trial [or endpoint]), there was a statistically significant interaction between medication and time. This interaction indicated a general difference in the efficacy among the four medications. To interpret this general difference, we did six pairwise post hoc tests of specific differences between treatments. Analogous analysis was performed for the 8-week period (results available on request).
Positive and Negative Syndrome Scale data at 14 weeks indicated statistically significant improvements in total score for the three atypical antipsychotics, improvements in negative symptoms and general psychopathology for clozapine, and improvements in positive symptoms and general psychopathology for olanzapine.
Clozapine and olanzapine were superior to haloperidol in terms of improvement on total score and negative symptoms. Haloperidol had no statistically significant effects on any Positive and Negative Syndrome Scale measure.
The data at 8 weeks (
Table 1) exhibit the last observations carried forward from the period of week 1 to week 8; observations beyond week 8 were censored. These 8-week data, which show trends similar to the entire 14-week trial, are displayed because that time point marks the end of the fixed-dose part of the study. Furthermore, this display facilitates comparisons with studies ending at 8 weeks
(8) or earlier. These ancillary results were corrected for test multiplicity in a way analogous to the results for the entire 14-week study. Symptom improvements were clinically modest. A standard measure for effect size (Cohen’s d
[17]) was adopted to describe improvement produced by each of the four drugs in statistical terms. The effect size index for each group was expressed as the ratio of the mean absolute improvement to the standard deviation within group. The effect sizes of the improvement in total score on the Positive and Negative Syndrome Scale at 14 weeks for clozapine, olanzapine, risperidone, and haloperidol were 0.33, 0.51, 0.18, and 0.11, respectively. As expected in a study group selected for suboptimal response to treatment with typical antipsychotics, haloperidol had no beneficial effects.
Since the olanzapine arm was added after the study had run for approximately 15 months, we considered the possibility of a cohort effect confounding our data. To explore this, we classified the patients in the clozapine, risperidone, and haloperidol arms (N=118) into two cohorts: those started before (N=68) and after (N=50) the introduction of the olanzapine treatment arm. The difference in Positive and Negative Syndrome Scale total score between baseline and endpoint was tested. An ANOVA showed no cohort effect (F=0.22, df=1, 117, p=0.64) or a cohort-by-medication interaction (F=0.51, df=1, 116, p=0.60). For subjects who began the study before and after the introduction of the olanzapine arm, the mean baseline severity (Positive and Negative Syndrome Scale total score) was 91.4 (SD=13.8) and 94.1 (SD=15.9), respectively. The mean overall improvement over the entire 14-week trial was 2.7 (SD=19.6) for those who started the study before olanzapine was added and 5.5 (SD=15.6) for those who started after. To explore this difference, we computed the improvements separately for each drug. A cohort effect would be expected to affect equally all three medication groups. Mean improvements for those who started the study before and after the introduction of the olanzapine arm, respectively, were 6.48 (SD=22.00) and 7.05 (SD=18.69) for those given clozapine, –0.03 (SD=20.07) and 7.92 (SD=12.48) for those given risperidone, and 1.68 (SD=15.78) and 1.62 (SD=15.51) for those given haloperidol. Thus, although the cohort-by-medication interaction was not significant, we see that the slightly better overall effect for those who started the study after olanzapine was introduced was largely attributable to patients in one medication group (risperidone). Similar results were obtained for the positive symptoms, negative symptoms, and general psychopathology subscales. Thus, although we cannot prove that there was no overall cohort effect, these and other computations (available on request) failed to detect it.
Since negative symptoms may be in part secondary to extrapyramidal side effects
(18), we repeated the hierarchical linear modeling analyses of negative symptoms with the Extrapyramidal Symptom Rating Scale total score as a covariate. The results of the analyses were not substantially affected by this covariate. Thus, the differences in treatment effects on negative symptoms were not mediated by those extrapyramidal side effects that are measured by the Extrapyramidal Symptom Rating Scale.
For clinical reasons, clozapine dose was escalated more slowly than the doses of the other medications. It was therefore possible that the aforementioned analyses did not adequately assess the effect of clozapine; some of the last observations in the clozapine group were perhaps obtained while the patients had not yet reached their full therapeutic doses. To explore this issue, we repeated the efficacy analyses for the subset of patients who completed the first 4 weeks of the study. (Thus, this was not a set of last observations carried forward). There were 33 patients receiving clozapine, 33 receiving olanzapine, 35 receiving risperidone, and 32 receiving haloperidol. At 4 weeks, the average daily dose of clozapine in these 33 patients was 453 mg (SD=66.6). The results of the hierarchical linear modeling analysis of these data indicated a statistically significant change over time for the main efficacy measure (overall time effect for Positive and Negative Syndrome Scale total score: F=26.2, df=1, 129, p<0.0001). The interaction between medication and time reached statistical significance (F=3.32, df=3, 963, p<0.02). The tests of fixed effects of time for each group were significant for clozapine (t=–4.3, df=963, p<0.0001), olanzapine (t=–4.2, df=963, p<0.0001), and risperidone (t=–2.0, df=963, p<0.04). Post hoc pairwise comparisons revealed superiority of clozapine over haloperidol (t=–2.8, df=963, p<0.006) and of olanzapine over haloperidol (t=–2.6, df=963, p<0.01). Thus, the results in this subset of patients who completed at least 4 weeks were essentially the same as those reported for the entire set at 14 weeks. Analogous analyses were performed for the positive, negative, and general psychopathology subscales of the Positive and Negative Syndrome Scale; these analyses essentially again replicated the results obtained in the complete set at 14 weeks. Thus, these analyses provided no support for the notion that the slower escalation rate of clozapine confounded the principal results.
Extrapyramidal Symptoms
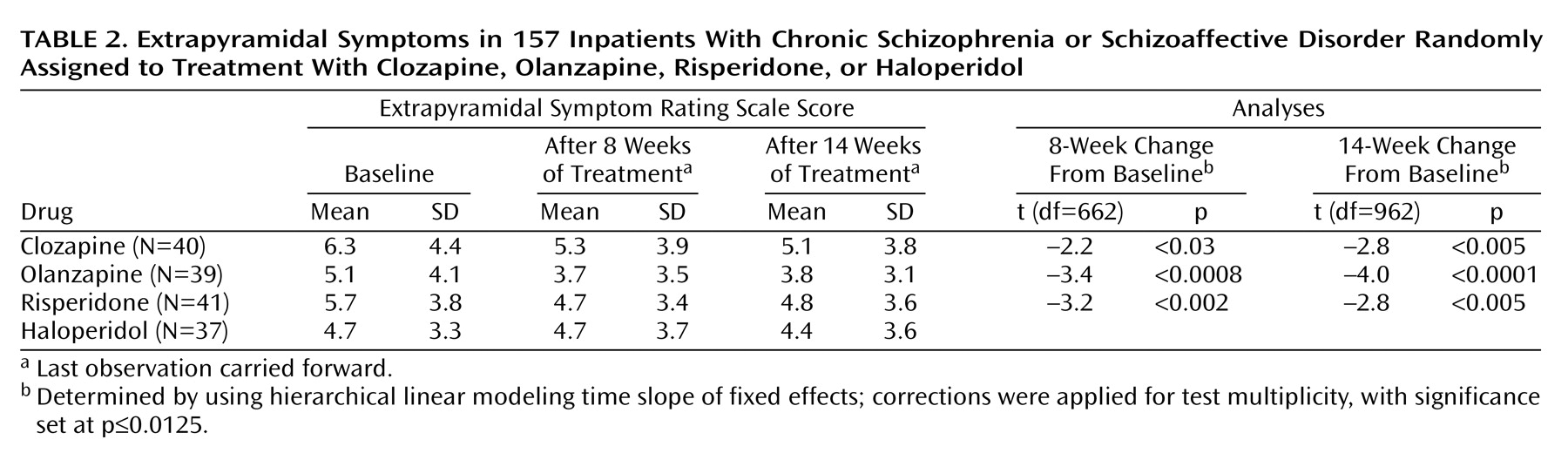
The hierarchical linear modeling analysis of fixed effects for extrapyramidal symptoms showed a significant time effect for both the 8-week (F=22.8, df=1, 152, p=0.0001) and 14-week data (F=24.1, df=1, 152, p=0.0001) but no significant interaction between time and medication. Decreases in scores on the Extrapyramidal Symptom Rating Scale occurred with each of the atypical antipsychotics (
Table 2). No significant results were detected for the total Extrapyramidal Symptom Rating Scale score, dyskinesia, or akathisia. Similar to the data in
Table 1, the tests are corrected for multiplicity.
Benztropine was administered prophylactically to all patients assigned to haloperidol, but for patients receiving the three atypical antipsychotics it was prescribed only if needed. The prescribers were blind as to the antipsychotic treatment assignment; benztropine usage could thus be employed as a variable reflecting the prescribers’ perception of the severity of the extrapyramidal side effects. We determined the number of patients assigned to atypical antipsychotics receiving benztropine after the first 2 weeks of the study. (The first 2 weeks were excluded in order to minimize carryover effects from prestudy treatments.)
The 8-week data indicate that benztropine was prescribed for 7.5% (N=3 of 40) of the patients receiving clozapine, 5.1% (N=2 of 39) of those receiving olanzapine, and 29.3% (N=12 of 41) of those receiving risperidone (χ2=11.8, df=2, p=0.003). The data for the entire 14 weeks were similar: benztropine was prescribed for 12.5% (N=5) of the patients receiving clozapine, 12.8% (N=5) of those receiving olanzapine, and 31.7% (N=13) of those receiving risperidone (χ2=6.32, df=2, p=0.04). Thus, benztropine prescriptions were significantly more frequent among patients assigned to risperidone, reflecting higher perceived frequency or severity of extrapyramidal side effects in the risperidone group.