Olanzapine, risperidone, quetiapine, and valproate provide effective treatment of bipolar disorder and psychosis
(1). Their use, however, is associated with weight gain in as many as 50% of patients
(1–
5). Metformin, which is prescribed for patients with non-insulin-dependent diabetes to control blood sugar levels, has been reported to effect weight loss in several groups of patients characterized by insulin resistance. These include women with polycystic ovary disease
(6,
7) and obese hyperlipidemic men and women with and without non-insulin-dependent diabetes
(8). Metformin has recently been approved for use in children. Within this framework, we conducted an open-label study of metformin and weight loss in pediatric patients who had gained weight while taking target drugs. We hypothesized that metformin would manage weight gain and lead to weight loss.
Method
Patients taking olanzapine, risperidone, quetiapine, or valproate at Children’s Hospital Medical Center, Cincinnati, with weight gain over 10% of baseline were given metformin, 500 mg t.i.d. Determination of weight gain was based on hospital records and the patient’s weight at enrollment. Weight and height were measured to the nearest 0.1 kg and 0.1 cm, respectively, according to a National Institutes of Health protocol
(9). The first two patients were seen under an 8-week protocol; subsequent patients were seen under a 12-week protocol. The patients were instructed not to change diet or physical activity during the study. Safety monitoring tests for lactic acidosis and liver function were conducted at all visits. Postmenarchal girls took urine pregnancy tests monthly.
The study’s primary outcome measures were change in weight and body mass index at 12 weeks of metformin treatment. For these outcomes, we used paired t tests to assess whether the mean changes were significantly different from zero. To control for multiple comparisons, we adjusted the critical alpha level by using Bonferroni adjustment, testing each outcome at an alpha of 0.025. We tested changes in weight and body mass index at 4 and 8 weeks as secondary outcomes, using a more conservative alpha of 0.01. All analyses were performed by using SAS version 8.0
(10).
The Children’s Hospital Medical Center institutional review board approved the protocol. Written informed assent/consent was obtained. The participants were told that we wanted to see whether metformin affected weight loss independently of dietary changes or physical activity.
Results
Nineteen patients, aged 10–18 years (mean=14.1, SD=2.5), were treated. They included 15 white and four black subjects; 12 were male, and seven were female. Two patients were taking valproate only, four were taking valproate plus olanzapine, eight were taking olanzapine only, one was taking valproate plus risperidone, three were taking risperidone only, and one was taking quetiapine only. The length of treatment with these drugs ranged from 1 to 36 months (mean=10.4, SD=9.5), and weight gain ranged from 6.0 to 59.1 kg (mean=28.6, SD=15.3). Follow-up data were available for 19 patients at 4 weeks, 16 patients at 8 weeks, and 12 patients at 12 weeks. Seven patients had less than 12 weeks of data. Two patients were seen under an 8-week protocol. One patient stopped taking olanzapine after 8 weeks of weight loss and was lost to follow-up, although later admitted to the hospital. One patient moved away. One patient, who gained 0.6 kg in 4 weeks, dropped out to try a different, dietary approach and was counted as gaining weight. Two patients did not complete the 12-week protocol before funding and institutional review board approval expired. The weight change outcomes were based on patients’ results at 4, 8, and 12 weeks, and the weight change classification was based on each patient’s final visit, whether that was at 4, 8, or 12 weeks.
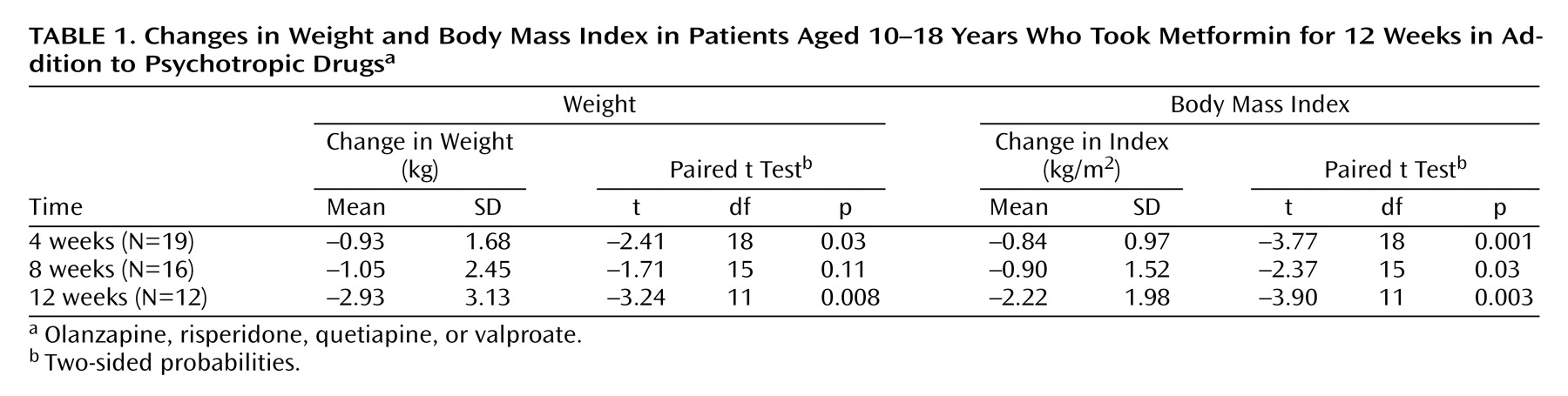
Of the 19 patients, 15 lost weight, the weight of one remained unchanged, and three gained weight. Weight gains in these three patients were 0.2 kg (over 3 months), 0.6 kg (over 1 month), and 1.6 kg (over 3 months). The mean changes in weight and body mass index by length of follow-up are summarized in
Table 1. At 12 weeks, the mean weight change was –2.93 kg, and the mean change in body mass index was –2.22 kg/m
2. Thus, both primary outcomes yielded highly significant results. Although there was weight loss and reduction in body mass index at weeks 4 and 8, only the reduction of body mass index at 4 weeks reached significance at the adjusted alpha level of 0.01 (
Table 1). Four patients followed as private patients beyond the 12-week protocol to evaluate longer-term weight loss continued to lose weight, achieving losses of 4.1, 4.6, 8.2, and 13.1 kg.
The results of the safety tests for lactic acidosis were unremarkable. That is, the readings outside the normal range were within laboratory error. Many of the patients with abnormal results on the liver function tests had abnormal readings at baseline. One of the two patients with low SGOT levels had a low level at baseline. Of the 10 patients with high SGOT levels, six had high levels at baseline. Of the five patients with low SGPT levels, one had a low baseline level. Of the four patients with high SGPT levels, one had a high baseline level. Of the three patients with high γ-glutamyltransferase levels, two had high levels at baseline. All other laboratory results were within normal ranges.
Discussion
In this preliminary evaluation of metformin as a treatment for weight gain associated with psychotropic drugs, the steep increase in weight experienced from these drugs was arrested in all patients. In addition, 15 of 19 patients lost weight. One patient, experiencing a weight gain of 1.6 kg, was also receiving intramuscular medroxyprogesterone acetate, an agent independently associated with weight gain.
Lustig
(11) reported that use of metformin led to weight loss in eight obese adolescent girls who had failed to lose weight after caloric restriction. The mechanism by which metformin effects weight loss is not presently understood, but evidence is accumulating that it influences metabolism. Metformin is known to improve insulin sensitivity. Lenhard et al.
(12) found that metformin increased mitochondrial metabolism, stimulating aerobic respiration and mitochondrial β-oxidation.
This preliminary study has several limitations. First, it was an open-label study, and the open-label treatment of obesity is subject to the placebo effect. However, the pattern of sustained, continued weight loss suggests that the weight loss was not due to the placebo effect. Second, a small number of patients were enrolled, so comparisons of weight loss among the target drugs were not possible. Different mean levels of weight gain with the various target drugs have been reported in previous studies
(3,
5). Third, different doses of metformin were not evaluated. Fourth, data on the occurrence of side effects were not systematically collected. Six patients reported diarrhea or watery stools at one or more visits. Fifth, although the patients reported making no change in energy intake or output, as instructed, hard data on dietary intake and physical activity were not collected. Sixth, long-term follow-up to determine the clinical significance of the mean weight loss was not conducted. The continued follow up of four patients beyond 12 weeks suggests clinically significant weight loss.
In this sample of 19 patients, metformin was well tolerated. One additional patient enrolled in the study but withdrew in the first month because of diarrhea. This patient was mentally retarded, wore diapers, and required a 2-hour bus ride each weekday. The patients reporting sporadic diarrhea, consisting of loose stools and/or increased frequency, indicated that it was not a cause for discontinuation and resolved with time. The results of safety tests were unremarkable.
We conclude that metformin holds promise as a treatment for weight gain in pediatric patients taking psychotropic medications. Further studies, including randomized controlled trials and longer follow-up periods, are warranted and required before a final evaluation is possible.