Tourette’s syndrome is a complex neuropsychiatric disorder characterized by fluctuating motor and phonic tics that may result in marked impairment
(1). An autoimmune etiology has been suggested for at least some cases
(2–
5). The presence of tics or obsessive-compulsive symptoms has been linked to infection with group A β-hemolytic streptococci
(2,
3). The molecular mimicry hypothesis predicts that genetically vulnerable individuals produce antibodies against streptococci that recognize self-proteins within the central nervous system (CNS). Cross-reactivity results in compromised neuronal function.
Several reports have shown that autoantibodies are present in a subset of patients with Tourette’s syndrome
(2–
5). However, the presence of autoantibodies does not indicate causality. An animal model in which these autoantibodies reproduce symptoms of the disorder is an important step. A recent animal model for Tourette’s syndrome reported the induction of stereotypies in rats after intrastriatal infusions of serum from Tourette’s syndrome patients
(6). The present report extends these findings by 1) infusing sera from patients with Tourette’s syndrome into a striatal region associated with oral stereotypy and 2) including sera from patients with Tourette’s syndrome with low levels of autoantibodies for comparison.
Method
We previously demonstrated that antineural and antinuclear antibodies were present in sera from a subset of patients with Tourette’s syndrome
(5). In the present study, sera were selected on the basis of their rank order for either IgG or total antibodies against either nuclear or neural protein
(5). Sera at or above the 75th percentile were identified as having a high autoantibody level, while sera below the 75th percentile were identified as having a low autoantibody level.
The study subjects included 12 Tourette’s syndrome patients with high autoantibody levels (seven male subjects and five female subjects, age range=10–19 years, mean=14.4) and 12 Tourette’s syndrome patients with low autoantibody levels (nine male subjects and three female subjects, age range=14–40 years, mean=21.5). Twelve comparison subjects with sera with staining intensities below the 75th percentile for both antineural and antinuclear autoantibodies were also selected (six male subjects and six female subjects, age range=11–35 years, mean=18.2). No subjects were taking psychostimulants at the time that blood was drawn. Twenty-seven subjects were taking no medication, six were taking neuroleptics, and three were taking clonidine. The effect of medication on stereotypies was assessed, and no differences between groups were found (Kruskal-Wallis H=0.95, df=2, p=0.62). Evidence for recent exposure to streptococcal infection was also assessed. No differences were found across the three groups for levels of antistreptolysin-O titers (F=1.21, df=2, 33, p=0.26) and anti-deoxyribonuclease-B titers (F=1.54, df=2, 33, p=0.23).
The ventrolateral striatum was selected on the basis of previous findings demonstrating that amphetamine infusion into this striatal subregion selectively produced oral stereotypies
(7,
8). Male Sprague-Dawley rats (275 g) were deeply anesthetized with pentobarbital/chloral hydrate anesthesia (equithesin) (4.5 mg/kg i.p.), and 28 gauge cannulae were implanted into each striatum by using standard surgical procedures
(9). Coordinates were anterior-posterior 2.0 mm from the bregma and medial-lateral ±4.0 mm and dorsoventral –7.0 mm from the skull
(10). Procedures were approved by the Animal Care Committee at Yale University.
The animals recovered for 1 week before being implanted with two osmotic mini pumps. Pumps were filled with phosphate-buffered saline and attached to cannulae by a polyethylene tube loaded with 50 μl of serum. Sera were not mixed together. Sera were adjusted to an IgG concentration of 2 μg/μl and infused at a rate of 0.5 μl/hour. Cannulae placement was confirmed by subsequent tissue examination. Inflammation was examined by hematoxylin and eosin staining. Slight inflammations were observed around the site of the cannulae, but no significant differences were detected between groups. One rat died due to anesthesia complications before infusion, leaving 11 rats available for analysis in the group receiving sera from the Tourette’s syndrome patients with low autoantibody levels.
Behavioral recordings began the day after infusion and continued for 5 days. Animals were observed during 30-minute sessions by two trained observers who were blind to the experimental condition. Observations were made every 5 minutes for 1-minute periods, for a total of six periods. Several categories of oral stereotypies, including wood chip eating, self-gnawing, biting, licking not associated with grooming, and “taffy pulling” (i.e., repetitive paw-to-mouth movements), were recorded. A rat that engaged in one or more episodes of the same behavior received a score of 1. Animals that exhibited one or more behaviors in any other categories received an additional point for each category observed. Scores ranging from 0 to 5 were averaged for each group (daily oral stereotypy score). In addition, a total oral stereotypy score for each group across the 5-day study period was calculated. This method has been used extensively to map striatal amphetamine-induced stereotypies
(7,
8). Behavioral data were analyzed by using nonparametric analyses of variance (Kruskal-Wallis test and Spearman rank-order correlation).
Results
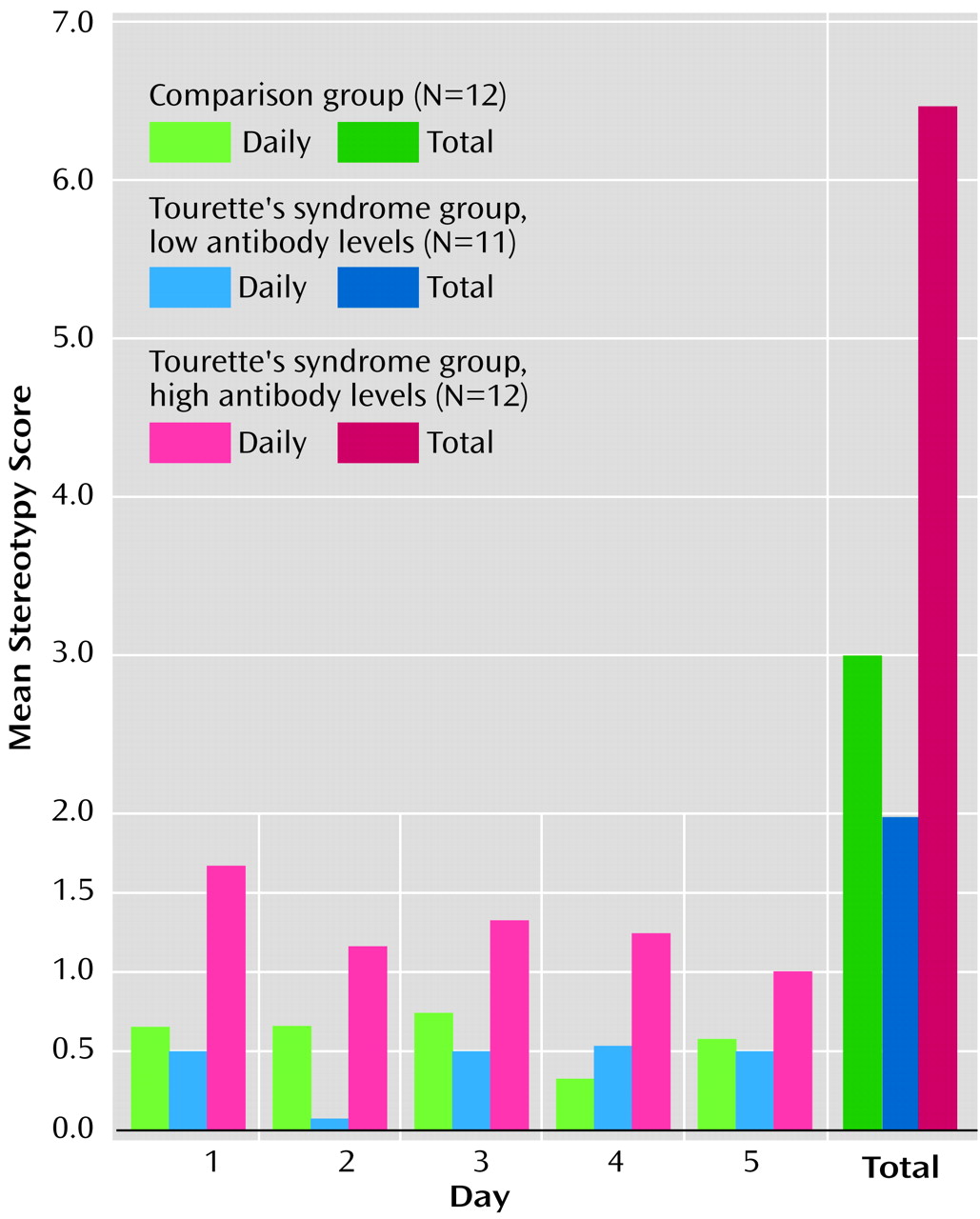
Infusions of sera into the ventrolateral striatum produced significant differences in both total and daily oral stereotypy scores. Total oral stereotypy scores were significantly higher in experimental animals (mean=6.4, SD=2.5), compared to animals infused with sera from normal subjects (mean=3.0, SD=1.9) or patients with Tourette’s syndrome with low autoantibody titers (mean=2.0, SD=2.2) (Kruskal-Wallis H=13.42, df=2, p<0.001) (
Figure 1).
Although the daily oral stereotypy score in the high-antibody Tourette’s syndrome group was significantly different from that in the other groups on days 1–4, the difference diminished by the fifth day of observation (day 1: high-antibody group, mean=1.7, SD=1.1; low-antibody group, mean=0.5, SD=0.7; comparison group, mean=0.7, SD=0.8; Kruskal-Wallis H=8.66, df=2, p<0.01) (day 2: high-antibody group, mean=1.2, SD=0.8; low-antibody group, mean=0.1, SD=0.3; comparison group, mean=0.7, SD=0.7; H=9.45, df=2, p<0.01) (day 3: high-antibody group, mean=1.3, SD=0.7; low-antibody group, mean=0.5, SD=1.2; comparison group, mean=0.8, SD=0.6; H=9.58, df=2, p<0.01) (day 4: high-antibody group, mean=1.3, SD=1.0; low-antibody group, mean=0.5, SD=0.8; comparison group, mean=0.3, SD=0.5; H=5.70, df=2, p<0.05) (day 5: high-antibody group, mean=1.0, SD=0.0; low-antibody group, mean=0.5, SD=0.7; comparison group, mean=0.6, SD=0.7; H=1.64, df=2, p<0.44). There were no significant differences in daily or total oral stereotypy between the comparison group and the low-antibody Tourette’s syndrome group. A positive correlation was found between the degree of oral stereotypic behavior and the extent of antinuclear IgG antibodies (rs=0.42, df=33, p=0.01) and antineural IgG antibodies (rs=0.43, df=33, p=0.02).
Although the present study focused on oral stereotypy, other behaviors were also altered. For example, there was a marked increase in genital grooming in the rats infused with high-antibody Tourette’s syndrome sera, compared with the other groups (high-antibody Tourette’s syndrome group: mean genital grooming score=4.0, SD=2.2; normal comparison group: mean=1.0, SD=0.0; low-antibody Tourette’s syndrome group: mean=0.9, SD=1.5) (H=6.52, df=2, p<0.03).
Discussion
The induction of oral stereotypies in rats infused with sera from patients with Tourette’s syndrome with high levels of autoantibodies is a striking finding. The results confirm and extend those of Hallett and colleagues
(6). Infusions into a striatal subregion associated with oral stereotypy produced highly significant effects as opposed to the weaker effects of infusions into the dorsolateral region of the striatum
(6), a region not typically associated with stimulant-induced oral stereotypy
(8). These findings extend earlier observations by selecting study groups on the basis of immunocytochemical analyses
(5). Moreover, the results for infusion of sera from Tourette’s syndrome patients with low levels of autoantibodies suggests that it is the presence of autoantibodies or other serum factor(s) that produces the observed stereotypies. The data are consistent with the molecular mimicry hypothesis proposed for etiology of a subgroup of Tourette’s syndrome cases.
A number of autoimmune disorders are caused by humoral factors. This mechanism has also been proposed for a subset of subjects with Tourette’s syndrome and obsessive-compulsive disorder (OCD)
(1–
6). The therapeutic benefit of removing autoantibodies was suggested as the mechanism for the positive response to plasmapheresis in individuals with poststreptococcal-associated Tourette’s syndrome or OCD
(11). In the present study, however, none of the six Tourette’s syndrome patients with high levels of antibodies for whom adequate data were available (pediatric records, extensive parental interviews with the National Institute of Mental Health forms for assessment of pediatric autoimmune neuropsychiatric disorders associated with streptococcal infections [PANDAS]) met the diagnostic criteria for PANDAS.
Animal models are a potential tool for developing or testing novel pharmacological therapies for Tourette’s syndrome. They may also facilitate the study of genetic and environmental factors that contribute to the expression of tics. One proposed mechanism for serum-induced stereotypies is that immunoglobulins recognize specific neuronal epitopes within the striatum and interfere with normal neuronal signaling, either alone or in conjunction with other serum factors. The immunofluorescent labeling pattern of the infused sera within the CNS from this study is being analyzed. Ongoing work will also determine whether immunoglobulins alone or some other serum factor(s) are responsible for the observed behavioral changes.