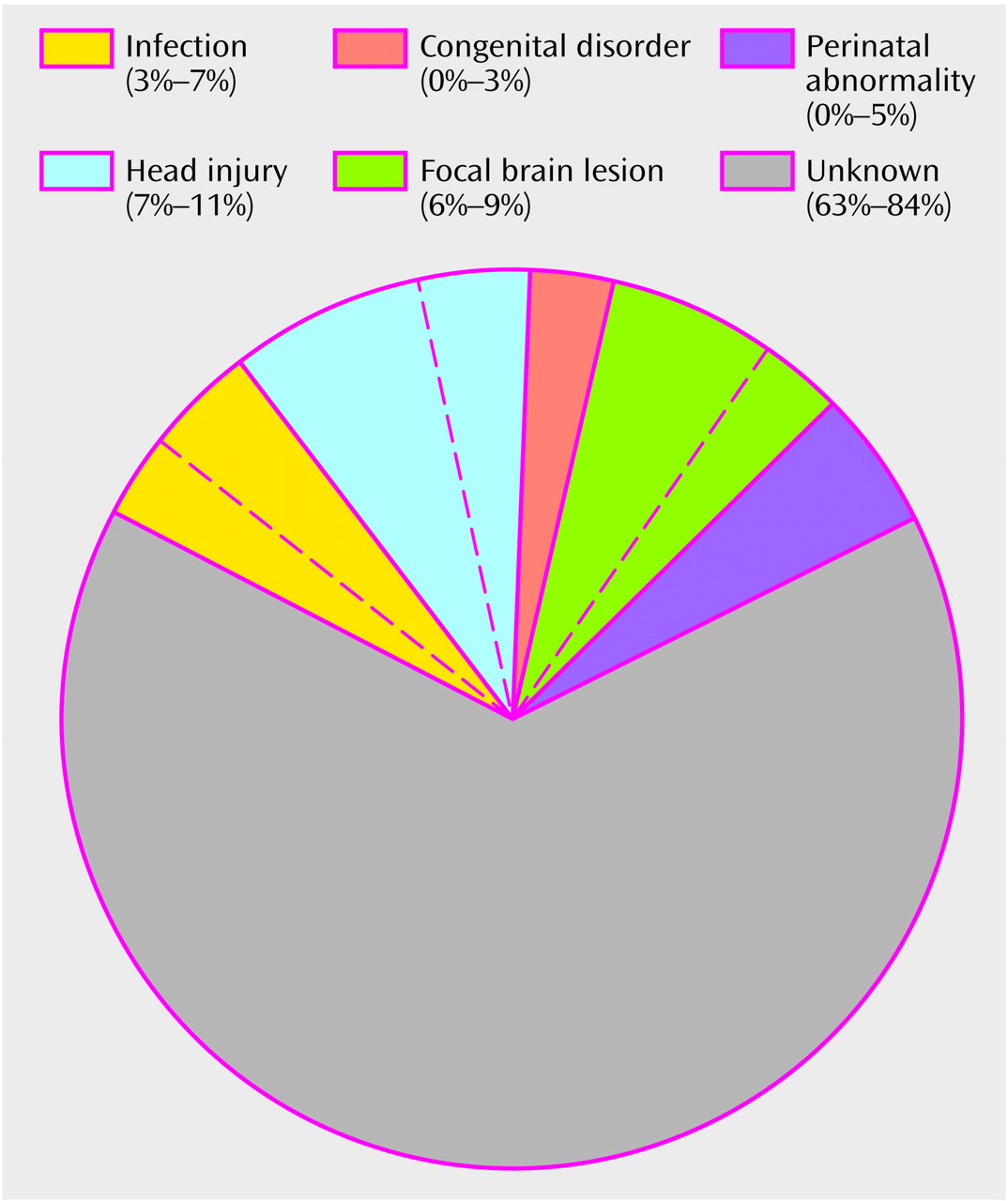
The etiology of schizophrenia remains unknown in a majority of cases (
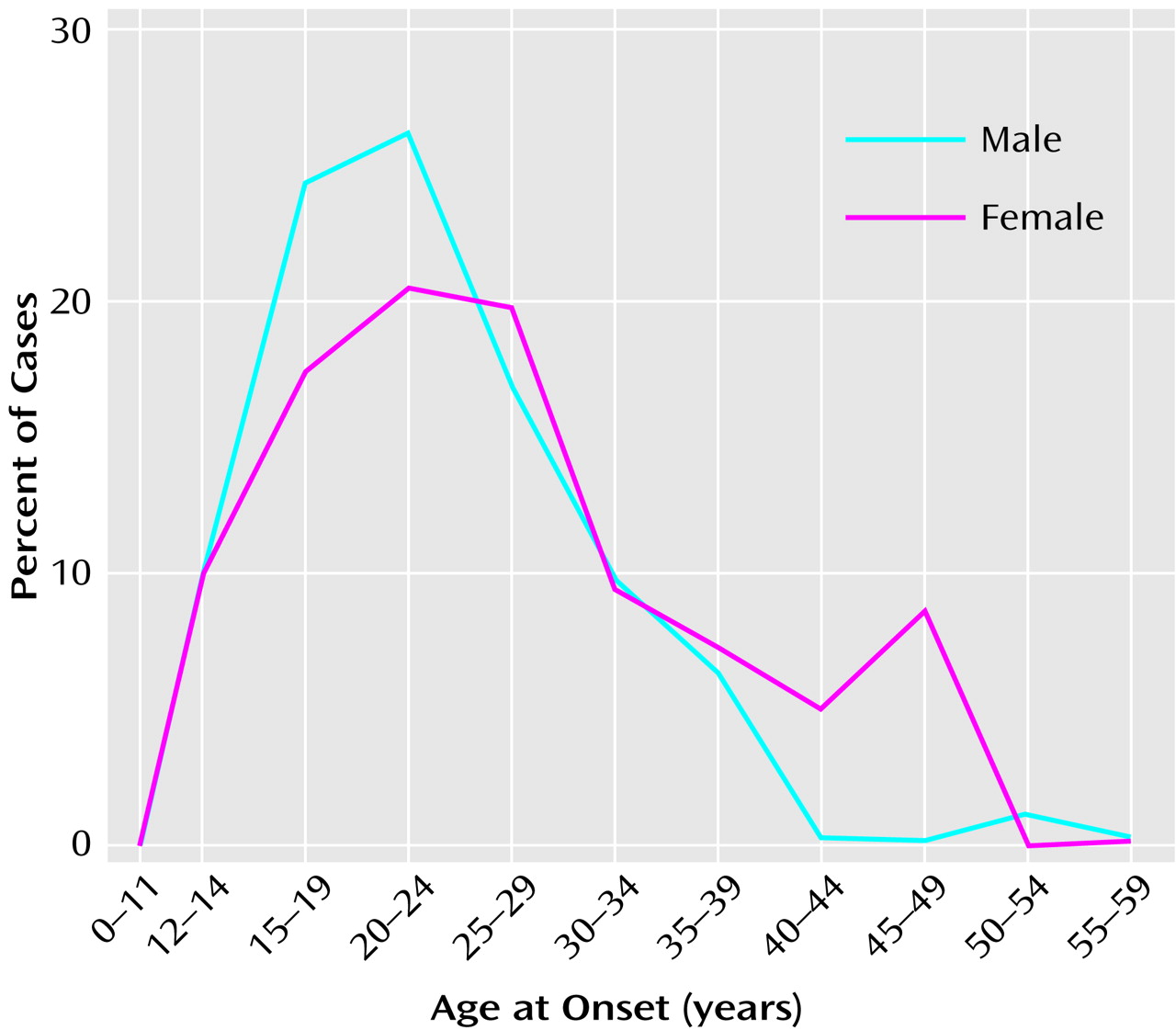
Figure 1). An important clue to the genesis of this disorder is the distribution of ages at onset, which closely parallels the ages at onset and decline of the reproductive period
(3) (
Figure 2). Between childhood and adulthood, pulsatile secretion of luteinizing hormone (LH), the hormone that stimulates gonadal growth and gonadal hormone secretion, increases more than 30-fold in boys and 100-fold in girls
(2). These hormonal changes are associated with the cascade of somatic, temperamental, and behavioral changes characteristic of puberty and of adult sexual and social behavior—and with the onset of schizophrenia in 0.5%–1% of the population. During the onset and duration of the reproductive period, circulation of the generally neuroexcitatory reproductive hormones to the brain and to the body requires compensatory changes in neurophysiology to augment the action of specific brain inhibitory systems or to diminish the action of endocrine excitatory systems. Failure to establish such an equilibrium is likely to be pertinent to the precipitation of schizophrenia in susceptible individuals.
A second clue is that, although whole brain weight is within normal limits in a majority of individuals with a diagnosis of schizophrenia, 25%–35% of those so diagnosed have larger than normal lateral ventricles and more than 50% have a larger than normal third ventricle and a lower than normal volume and number of neurons or molecular markers in one or more thalamic nuclei
(3–
7). A significantly lower than normal volume of the cerebral gyri or cortex, generally in the frontal or temporal lobes, has been reported in 10%–15% of schizophrenic patients
(4). Statistically significant differences in the number of dendritic spines or intracellular or synaptic proteins are reported in subgroups of patients
(8). Since skull size is normal in the majority of patients with schizophrenia
(8), a lower than normal volume of brain tissue must in a majority of cases involve subcortical structures or, if cortical structures are involved, must occur after age 4–5 years, when head size reaches an adult level.
Treatment and Pathophysiology
The first effective modern treatment for schizophrenia was induction of epileptic seizures by pharmacologic convulsants or by electrical stimulation at the scalp
(14). Thus, it is not surprising that current effective treatment uses a variety of antipsychotics that antagonize cerebral receptors for neurotransmitters that are generally considered inhibitory, e.g., dopamine, serotonin (5-HT), norepinephrine, and γ-aminobutyric acid (GABA)
(15–
19). Pharmacologic blockade of receptors for each of these neurotransmitters can induce epileptic seizures in both animals and humans, depending on the dose of and individual susceptibility to the blocking agent. In contrast, schizophrenia-like psychoses may be precipitated in susceptible individuals by pharmacologic agents that enhance inhibitory factors, e.g., the excitatory amino acid antagonists phencyclidine, MK801, and monoamine agonists such as the amphetamines and lysergic acid diethylamide (LSD)
(20,
21).
The most effective agent against treatment-resistant symptoms of schizophrenia is the atypical antipsychotic clozapine
(22,
23). Unlike earlier antipsychotics, which have a high affinity for dopamine D
2 receptors, clozapine has greater affinity for the serotonin (5-HT
1A, 5-HT
2, 5-HT
3), muscarinic, histamine, dopamine D
3, and dopamine D
4 receptors and blocks
N-methyl-
d-aspartic acid antagonists
(18,
19). Although all neuroleptics can cause seizures in susceptible individuals or when given in high doses, clozapine is the most proconvulsive of these agents
(24). Clozapine blocks dopamine D
3 receptors in the nucleus accumbens more than in the caudate-putamen, and, in contrast to other antipsychotics, induces
c-fos expression in the accumbens shell but not in the caudate-putamen
(25). Clozapine does not produce the extrapyramidal side effects associated with the earlier antipsychotics and with high doses of the newer atypical antipsychotics
(16–
18). Higher levels of dopamine in the nucleus accumbens and the islands of Calleja
(26) and expression of dopamine D
3 receptors in the nucleus accumbens, the ventral striatal gateway from amygdala and hippocampus to thalamus and frontal lobes have been reported in brains from some schizophrenic patients
(27). In one recent report, a higher level of dopamine D
3 receptors was reported from peripheral blood lymphocytes of unmedicated schizophrenic patients as well as those receiving neuroleptics
(28). Polymorphisms of the dopamine D
3 receptor have been associated with schizophrenia
(29). Clozapine has greater affinity for 5-HT
2A and dopamine D
4than for dopamine D
3 receptors. However, in contrast to agents that block dopamine D
2 and dopamine D
3 receptors, 5-HT
2A and dopamine D
4 antagonists are not effective against symptoms of schizophrenia
(30). Unlike neuroleptics that principally block the dopamine D
2 receptor and cause a sustained and substantial increase in serum prolactin and Fos in the caudate-putamen, clozapine induces only a transient smaller increase in serum prolactin and elicits expression of Fos in the paraventricular nucleus of thalamus
(31) and expression of Fos and
c-fos mRNA in the islands of Calleja in the basal forebrain
(32). The possible significance of these phenomena will be discussed in the next section.
Schizophrenia and the Reproductive Circuits of the Brain
The onset of schizophrenia at the beginning of or during the reproductive period suggests a relationship between this disorder and the dramatic changes in the brain and the body that take place during adolescence and throughout the fertility period. During adolescence, these changes include activation and amplification of the pulsed release of gonadotropin-releasing (luteinizing) hormone, a 30-fold increase in release of LH in boys and a 100-fold increase in girls, and a rapid increase in circulating estrogens and androgens from the gonads
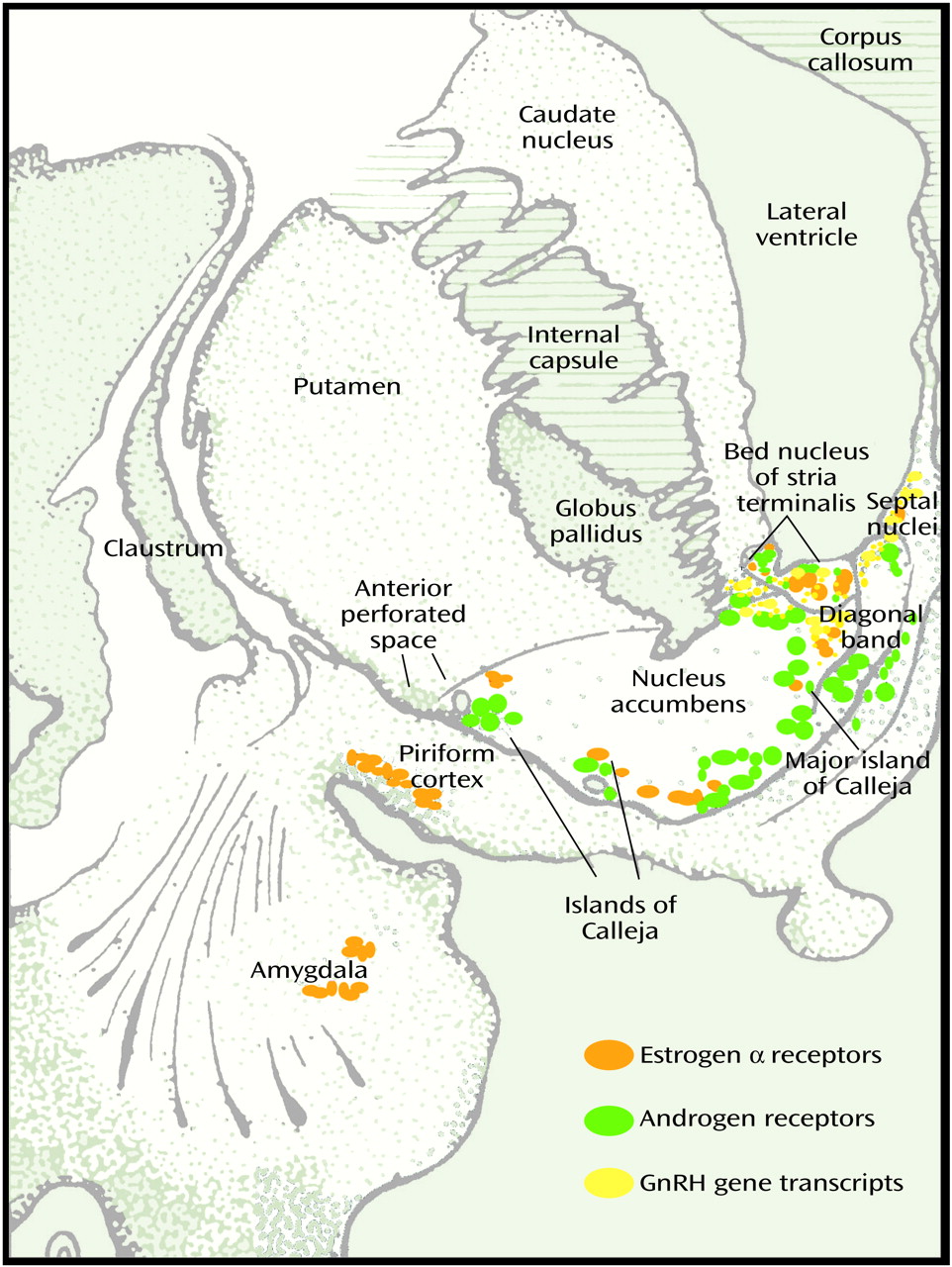
(2). In addition to being present in the hypothalamus, androgen and estrogen receptors in the brain are concentrated in the medial amygdala, bed nucleus of stria terminalis, and lateral septum in the basal forebrain of all mammals including humans (
Figure 3 [33]). Steroid-induced changes in the limbic-hypothalamic circuit during the reproductive cycle have been reported by several investigators. Woolley and McEwen (34) reported a change in the numbers of dendritic spines on pyramidal neurons in the highly epileptogenic CA1 area of the hippocampus of the rat in response to estrogen. The increase in circulating androgens and estrogens associated with sexual maturation leads to other significant changes in brain axons and receptors as well as the well-known physical, physiologic, psychologic, and behavioral changes associated with the flood of hormones to brain and body during this period
(35,
36).
Between the ages of 9 and 13 years, the pulsatile release of gonadotropin-releasing hormone is associated with regular episodic bursts of neuronal discharge in the arcuate nucleus of the hypothalamus reflected in the episodic release of LH to the bloodstream from the anterior pituitary
(2). Episodic neuronal discharges also occur in the amygdala, hippocampus, bed nucleus of stria terminalis, and septal and other limbic nuclei during coition, ovulation, and other critical events of the reproductive cycle
(37–
41). The physiologic occurrence of these sharply localized high-frequency discharges in limbic forebrain structures that concentrate and express receptors for reproductive hormones indicates the importance of brief synchronous bursts of rapid neuronal activity and of the limitation of such bursts to circumscribed areas for delivery of vital messages in the neuroendocrine network.
The amygdala and hippocampus, specific regions of which project to these basal forebrain structures and to the hypothalamus, are normally endowed with the lowest threshold for rapid neuronal seizure-like discharge of any brain region. This fact requires that excitatory circuits (principally glutamatergic and cholinergic axon terminals that project from the excitatory neurons of the amygdala and hippocampus to the nucleus accumbens, septum, and other forebrain sites) be protected by inhibitory networks to prevent propagation of episodic physiologic rapid discharges outside areas of physiologic utility, leading to seizures. Inhibitory activity is provided by the high concentration of dopamine D
3, 5-HT, and GABA-ergic terminals and receptors in this region. The action of these inhibitory systems must be increased to balance the effect of the entry of the excitatory reproductive hormones into specific brain areas at puberty and throughout the reproductive period. However, excessive inhibitory response to these physiologic events via dopamine, 5-HT, or GABA can cause psychosis in susceptible individuals. Indeed, the psychotic response to potentiators of inhibition such as amphetamine and ketamine does not occur before puberty
(20,
42). Very few published studies have examined the changes in innervation of the brain that occur during and after the onset of puberty and the reproductive period (B. McEwen, personal communication, 2001). However, Benes et al.
(43) reported a progressive increase in myelination in the subiculum and presubiculum of the hippocampus during normal human maturation, and Fink et al.
(44) described a marked increase in 5 HT
2A receptors in the forebrain coincident with proestrus in the rat. The importance of gonadal hormones in modulating the dopamine system is dramatically illustrated by the permanent loss of 30% of dopamine neurons from the substantia nigra after oophorectomy in the monkey
(45).
In the brain of both male and female subjects, estrogen α and β receptors and progesterone, androgen, and prolactin receptors are widely distributed in several extrahypothalamic basal forebrain sites, including the amygdala, lateral septum, bed nucleus of stria terminalis, nucleus of diagonal band, basal nucleus of Meynert, periaqueductal grey matter, and islands of Calleja
(46–
50) (
Figure 3). In addition to receptors for LH and for estrogen and testosterone, gonadotropic-releasing hormone mRNA is present outside the hypothalamus in a number of basal forebrain and limbic structures, including the central and dorsal nuclei of amygdala, ventral striatum, ventral pallidum, putamen, basal nucleus of Meynert, bed nucleus of stria terminalis, and septal and dorsal preoptic regions in both rat and human
(51).
The islands of Calleja are multiple discrete clusters of small granular neurons in the ventral striatum surrounding larger pallidal-like neurons and a vascular core. Islands of Calleja complexes are present in all species and attain maximum development and dispersion in humans
(52). Surrounded by a dense plexus of cholinergic, dopaminergic, and peptidergic axons, the islands of Calleja complexes are embedded in the ventral and medial border of the ventral striatum (nucleus accumbens in primates, as olfactory tubercle has largely disappeared in primate species). The large island of Calleja (major) is located in the medial border of the accumbens adjacent to the vertical limb of the diagonal band (
Figure 3). Unlike the neurons in the neostriatum and ventral striatum, some of the larger neurons of the islands of Calleja contain gonadotropin-releasing hormone (luteinizing hormone-releasing hormone), concentrate estradiol, and express estrogen and testosterone receptors
(53–
56). The ventral group of islands of Calleja is dispersed along the pial border of the basal forebrain in close relation to blood vessels, suggesting possible humoral inputs, including serum endocrine factors
(53,
54). Axons from the large medial island of Calleja, in parallel to the ventral striatum, project to the dorsomedial nucleus of thalamus and could thus influence this relay nucleus to the frontal lobes. The islands of Calleja are surrounded by muscarinic and 5-HT terminals and contain the highest concentration of dopamine D
3 receptors in brain
(55,
56). In addition to antagonizing dopamine D
3 and 5-HT receptors, clozapine is a potent muscarinic antagonist and elicits Fos immunoreactivity in the islands of Calleja
(57).
Fallon and associates
(53,
54) proposed that the anterior basal forebrain network containing gonadotropic hormones and androgen and estrogen receptors constitutes an extrahypothalamic hormone regulatory system closely related to the sensory, motor, and affective aspects of reproductive behavior. A threefold increase in the pituitary hormone prolactin during sleep, abnormal growth hormone response to luteinizing hormone-releasing hormone and thyrotropin-releasing hormone, and a lower level of total gonadotropins and testosterone were reported in unmedicated male schizophrenic patients, relative to age-matched comparison subjects
(58,
59). Other investigators have attributed the later onset of schizophrenia in women and the postmenopausal onset of the disorder to the protective effect of estrogen
(3,
60,
61). Estrogen is excitatory in the brain, and changes in estrogen level during the menstrual cycle correlate with exacerbation or remission of psychosis or seizures in susceptible girls and women
(61,
62). Aromatase, which converts testosterone to estrogen, is widely distributed in the brain and other organs and may be of significance in schizophrenia
(63).
The extension of the sharply localized episodic and rapid neuronal discharges associated with endocrine release beyond regions of physiologic utility, which could lead to seizures, is opposed by widely distributed inhibitory pathways and transmitters. These include dopamine, norepinephrine, 5-HT, GABA, specific peptides, and the multiple receptors for these transmitters. Dopamine D
2 receptors are maximally expressed in the neostriatum (caudate-putamen) where excitatory (glutamate) axons from the neocortex and thalamus converge. Dopamine D
3 receptors predominate in the ventral striatum (nucleus accumbens in humans), where axons from the amygdala and hippocampus, the regions that physiologically demonstrate the lowest threshold for epileptic discharge in the brain, project. The accumbens projects to the ventral pallidum and then to the hypothalamus and to the dorsomedial thalamus and orbital and medial frontal lobes
(64,
65). Maximum expression of dopamine D
3 receptors in the islands of Calleja and nucleus accumbens is consistent with the required limitation of neuronal discharges from the physiologically seizure-prone amygdala and hippocampus from spreading beyond their projection sites in the hypothalamus, accumbens, septal, and related basal forebrain nuclei. The therapeutic as well as the epileptogenic propensity of clozapine may relate to preferential block of dopamine D
3 and 5-HT
2A receptors that are strongly expressed in these basal forebrain sites, which are rich in reproductive hormones and endocrine receptors.
The concentration of excitatory (muscarinic) and inhibitory (dopaminergic) plexuses enclosing islands of forebrain neurons that express receptors for reproductive hormones is consistent with a balance of inhibitory and excitatory activity that regulates the effect of the reproductive hormones on neuronal excitability in this region. Pharmacotherapeutic evidence for disturbed cerebral monoamine regulation in schizophrenia draws attention to this forebrain area. The surge of excitatory reproductive hormones to this area during the reproductive period must be counterbalanced by enhanced inhibitory activity to prevent seizures, but overcompensation may induce psychosis. Maximum occurrence of schizophrenia during the fertility period and the peculiar olfactory, tactile, and sexual hallucinations and delusions frequently experienced by individuals with this disorder may indicate abnormal augmentation of one or more inhibitory factors induced by the physiologic flood of excitatory gonadal steroids to the anterior basal forebrain areas where receptors for the excitatory reproductive hormones, olfactory centers, and projections to the thalamus and frontal lobes converge.
Other research has provided additional evidence of altered excitability of neurons in the basal forebrain in schizophrenia. Many years ago several groups of investigators reported episodic spike activity recorded from electrodes implanted in the septal region or amygdala of patients with active symptoms of schizophrenia
(10–
13). Similar single-spike activity is often observed in the EEGs of individuals with epilepsy recorded during periods between seizures. Multiple spikes resembling focal seizures were also recorded by EEG from the septal region of normal men during orgasm or during orgasmic sensations induced by electrical or cholinergic stimulation of this region
(10,
11). Rats with stimulating electrodes placed in the septal accumbens area will press a lever hundreds of times per minute for electric stimulation to the point of producing seizures
(66). Stimulation of septal-accumbens area is associated with positive reward in rats and orgasm in humans
(10,
11). The dense dopaminergic innervation and high concentration of dopamine D
3 receptors in the basal forebrain are consistent with an extrahypothalamic regulatory system that limits propagation of physiologic excitatory discharges critical to sexual and reproductive function from extending beyond areas of physiologic utility. The special value of clozapine for treatment of some otherwise treatment-resistant cases of schizophrenia could relate to the relatively greater affinity of this drug for these receptors, which are physiologically strongly expressed in the basal forebrain area and may be excessively expressed in schizophrenia
(26–
28) (as, for example, are several excitatory systems in epilepsy).