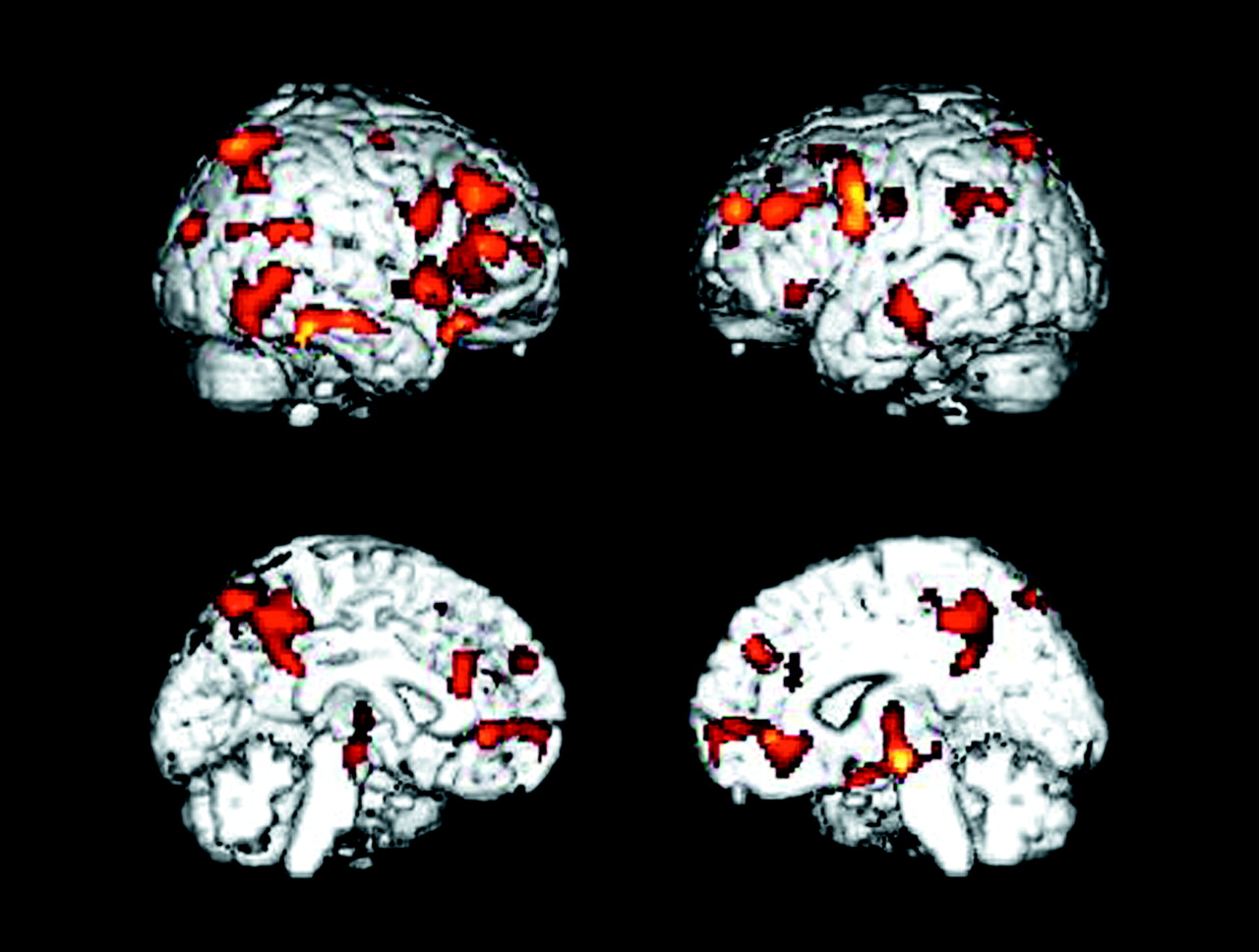
It is well established that [
18F]fluorodeoxyglucose (FDG) positron emission tomography (PET) assessed during a mental resting state with eyes covered and ears occluded provides a sensitive, in vivo metabolic index of the neurophysiological effects of Alzheimer’s disease. Numerous cross-sectional studies
(1–
5) have shown that cerebral metabolic rates in parietal and temporal association regions are most consistently and severely lower in patients with Alzheimer’s disease than in healthy comparison subjects and that frontal association regions are increasingly affected as the disease progresses. Further, studies have shown that the posterior cingulate cortex may be affected very early in the progression of Alzheimer’s disease
(6), even before the onset of clinical symptoms
(7). Relatively few studies, however, have directly evaluated the longitudinal effects of the dementia in Alzheimer’s disease using PET
(8–
11). The available longitudinal PET studies have typically relied on relatively large regions of interest to assess regional effects over time and have not applied more recently available brain mapping algorithms to investigate regional progression of Alzheimer’s disease throughout the brain. Applying such a voxel-based approach may offer greater regional specificity than region-of-interest methods in characterizing the distribution of maximal declines in cerebral metabolism in Alzheimer’s disease.
Studying the longitudinal effects of Alzheimer’s disease on brain function is particularly relevant given the current interest in developing treatments that can slow or halt its progression. Most treatment studies have relied on general cognitive tests and global behavioral ratings to evaluate treatment efficacy in patients with Alzheimer’s disease
(12,
13). Using functional neuroimaging to detect in vivo longitudinal changes in glucose metabolism directly in the brain may greatly enhance the ability to evaluate long-term treatments in the earliest stages of Alzheimer’s disease
(14,
15). Further, in vivo metabolic studies provide the chance to determine the extent to which clinically effective treatments attenuate or potentially compensate for disease progression. The feasibility of using PET to evaluate long-term treatment effects in dementia, however, has yet to be directly investigated.
In this study, we used FDG PET and a statistical brain mapping algorithm to investigate cross-sectional and longitudinal reductions in regional cerebral glucose metabolism on a voxel basis in 14 Alzheimer’s disease patients with mildly to moderately severe dementia studied at baseline and after a 1-year follow-up. The baseline PET scans in the patients with Alzheimer’s disease were compared with scans obtained in 34 healthy comparison subjects of similar age and sex. Power analyses for voxels showing maximal 1-year rates of decline in regional cerebral glucose metabolism and for annual decline rates in cognitive test scores were computed for the Alzheimer’s disease group.
We hypothesized that the patients with Alzheimer’s disease would show metabolic decline over 1 year in brain regions known to be affected by the progression of Alzheimer’s disease. By using a voxel-based approach for the PET data analysis, we sought to identify those specific brain areas showing maximal decline in the patients with Alzheimer’s disease and to test with statistical power analyses the relative feasibility of using these regional cerebral glucose metabolism values as a potential outcome measure in long-term treatment studies of dementia in Alzheimer’s disease.
Results
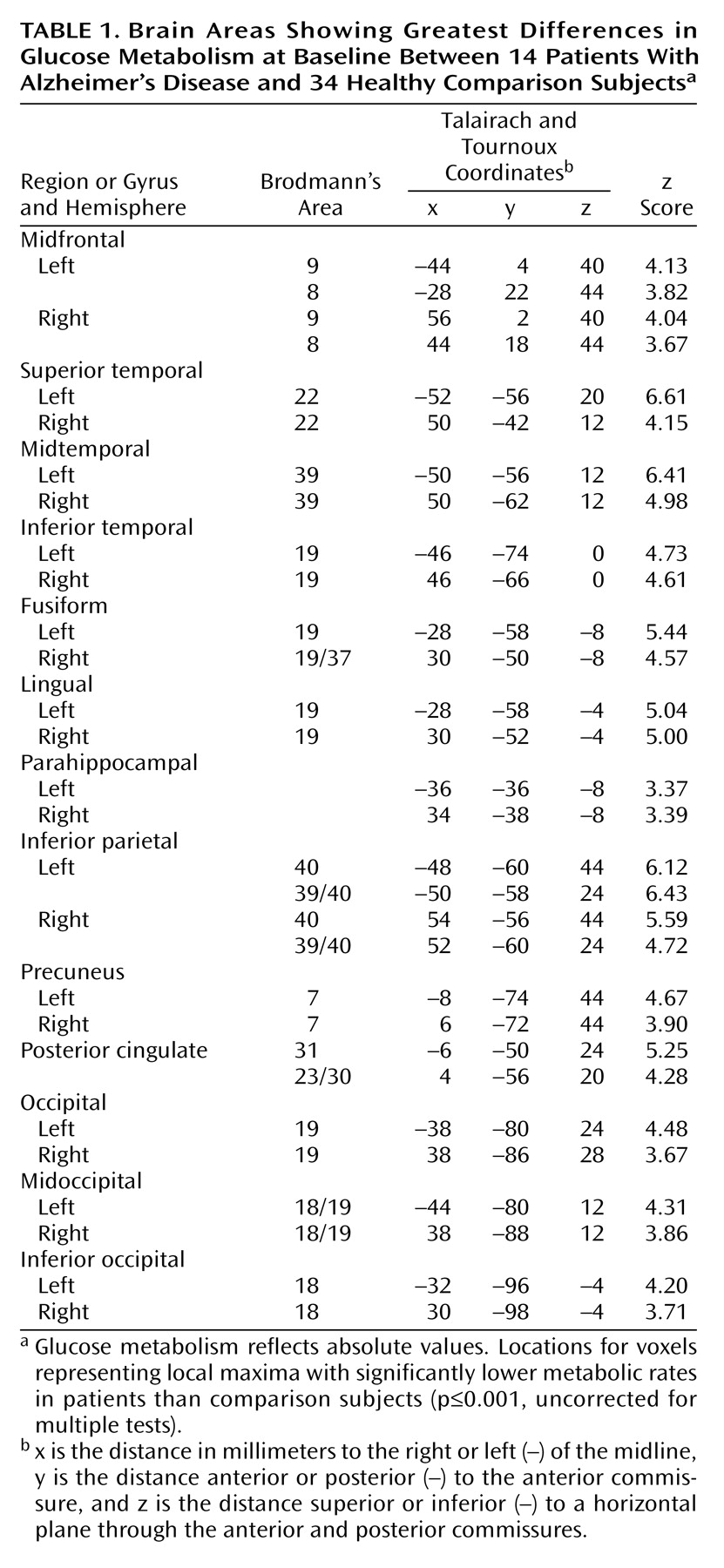
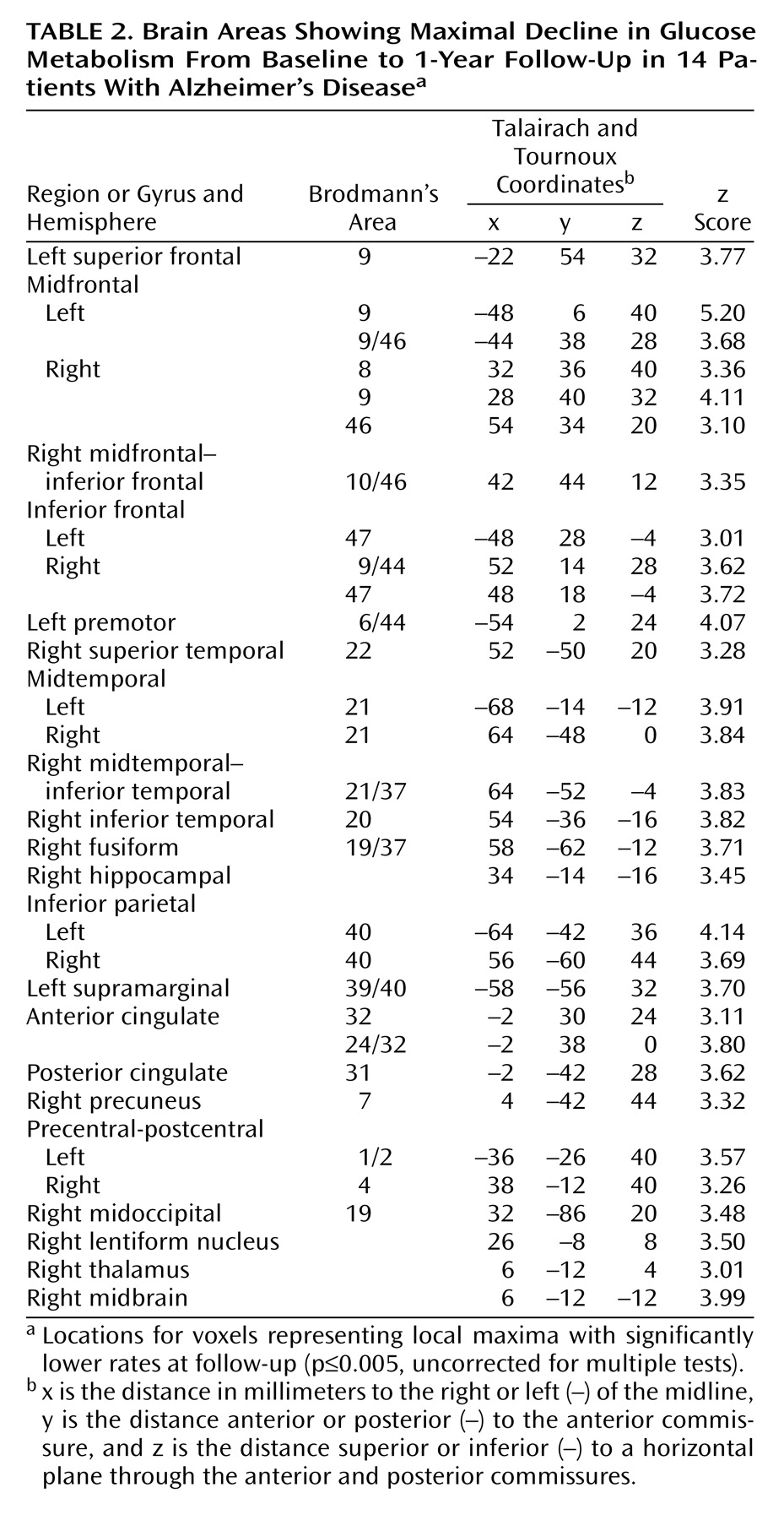
The patients with Alzheimer’s disease demonstrated significantly (p≤0.001, uncorrected for multiple tests) lower glucose metabolism than the healthy comparison subjects in multiple brain areas, including parietal, temporal, occipital, and frontal association regions and the posterior cingulate cortex (
Table 1 and
Figure 1). Metabolism in these regions was also significantly lower in patients than comparison subjects when the glucose metabolism values were ratio normalized to the pons (3.1≤z≤5.6).
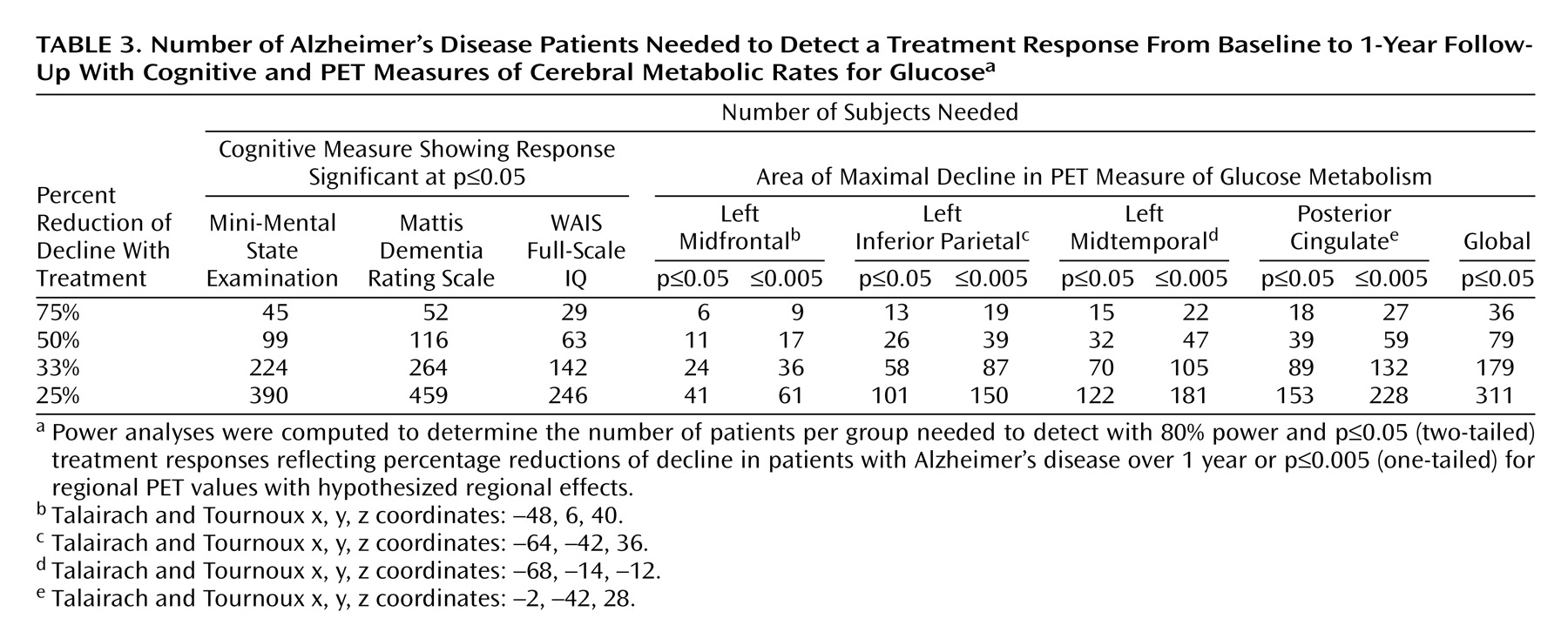
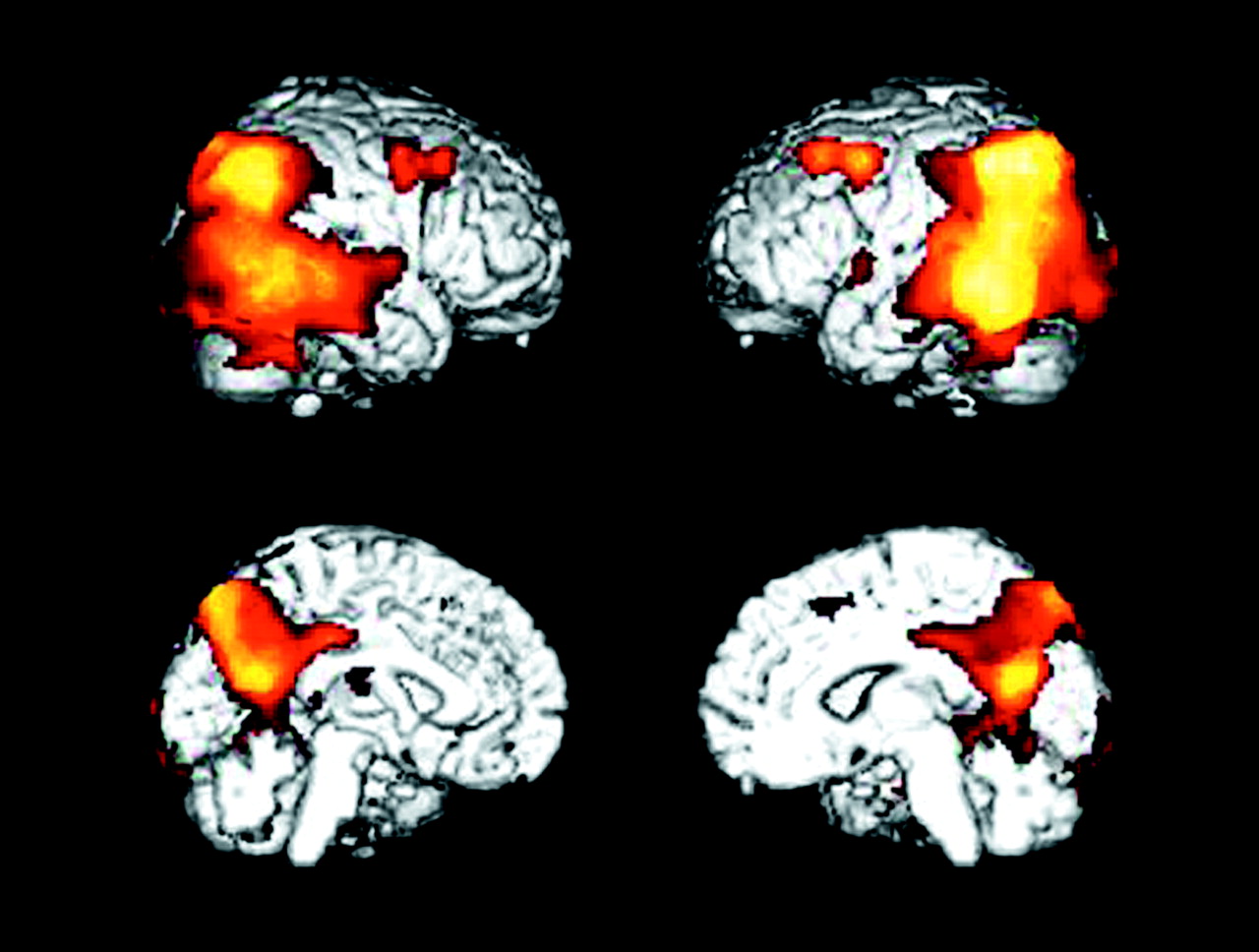
At 1-year follow-up, the patients with Alzheimer’s disease showed significant (p≤0.005, uncorrected for multiple tests) declines in glucose metabolism from baseline in the expected parietal, temporal, frontal, and posterior cingulate regions (
Table 2 and
Figure 2>). Declines were also observed for glucose metabolism values normalized to the pons in parietal and temporal regions (3.1≤z≤3.6), but these effects were generally diminished compared with declines in absolute glucose metabolism, and the decline in the frontal cortex did not reach significance.
The patients with Alzheimer’s disease showed a significant decline in MMSE scores over the 1-year interval (mean decline=2.9, SD=3.5) (t=3.05, df=13, p<0.01) as well as in Mattis Dementia Rating Scale scores (mean decline=18.1, SD=23.7) (t=2.86, df=13, p<0.014) and WAIS full-scale IQ (mean decline=9.7, SD=9.3) (t=3.90, df=13, p<0.002). Mean decline in glucose metabolism (mg/100 g per minute) for voxels showing maximal decline over the 1-year interval in the patients with Alzheimer’s disease was 0.86 (SD=0.33) in the left midfrontal cortex, 0.59 (SD=0.36) in the left inferior parietal region, 0.67 (SD=0.45) in the left midtemporal region, and 0.49 (SD=0.37) in the posterior cingulate cortex (
Table 2). The patients with Alzheimer’s disease also showed a significant decline from baseline in global mean cerebral glucose metabolism (mean decline=0.26, SD=0.28) (t=3.47, df=13, p<0.005).
Table 3 shows the results for power analyses performed with the general cognitive measures and absolute PET decline in regional cerebral glucose metabolism values. These analyses estimated the number of subjects needed to detect 75%, 50%, 33%, and 25% reductions of decline in a randomized, double-blind, placebo-controlled Alzheimer’s disease treatment study. The power analyses estimated that a PET study of glucose metabolism would require as few as 24 patients with Alzheimer’s disease per group when the voxel value for the left midfrontal cortex that showed maximal decline in 1 year was used and from 58 to 89 patients per group when voxels showing maximal declines in the left inferior parietal, left midtemporal, and posterior cingulate cortices were used to detect a significant (p≤0.05, two-tailed) 33% effect of treatment with 80% power compared with the effect of placebo. Using the global mean cerebral glucose metabolism value, the analysis estimated that 179 patients per group would be required to detect the same treatment response.
The power results for the general cognitive tests estimated that from 142 to 264 patients with Alzheimer’s disease per group would be required, depending on the test used, to demonstrate a 33% treatment response with 80% power and p≤0.05 (two-tailed) significance.
Since data analysis in regional PET studies with statistical parametric mapping on a voxel-by-voxel basis often uses more conservative significance thresholds, we recalculated the power analyses for the PET voxels showing maximal declines using p≤0.005 (one-tailed, uncorrected for multiple tests), because this threshold has been used for hypothesized regional effects in PET studies. These analyses estimated that as few as 36 patients with Alzheimer’s disease per group would be needed to detect a 33% treatment response with 80% power and p≤0.005 (one-tailed, uncorrected for multiple tests) (
Table 3).
Discussion
As expected, the Alzheimer’s disease group showed prominent cerebral glucose hypometabolism compared with healthy comparison subjects in bilateral parietal, temporal, and occipital association areas and the posterior cingulate cortex. Additionally, significant differences between patients and comparison subjects were observed at baseline in bilateral frontal association regions. These cross-sectional regional effects were present for both absolute glucose metabolism and values normalized to the pons and are consistent with previous reports of patterns of cerebral glucose hypometabolism typically observed in the mild to moderate stages of dementia in Alzheimer’s disease
(2–
5).
Evaluation of cerebral metabolic decline in the patients with Alzheimer’s disease after a 1-year follow-up showed additional significant reductions in absolute glucose metabolism from baseline in regions contained within the areas of hypometabolism initially observed in the cross-sectional analyses, including parietal, temporal, frontal, and posterior cingulate cortices. A voxel in the left midfrontal gyrus showed the greatest decline over 1 year. These findings support previous studies showing that patients with Alzheimer’s disease have preferential and progressive metabolism reductions in parietal, temporal, prefrontal, and posterior cingulate brain regions
(11). By using a voxel-based analysis, we were able to identify areas of maximal decline throughout the brain with greater regional specificity than has been described in longitudinal PET studies that have relied on relatively large regions of interest.
That we observed the most significant regional declines over the 1-year interval in the patients with Alzheimer’s disease when we applied absolute measures of glucose metabolism supports the value of quantitative PET for treatment studies in patients with dementia. In this study, we obtained quantitative measures of regional cerebral glucose metabolism with arterial blood sampling throughout the scan. Although full quantitation of PET has typically relied on this relatively invasive procedure, Chen et al.
(29) reported a relatively noninvasive method (i.e., not requiring an arterial line) to obtain a highly accurate quantitation of regional cerebral glucose metabolism with PET, making the use of quantitative regional cerebral glucose metabolism to monitor decline over time practical for large treatment studies of dementia.
By applying power analysis of glucose metabolism assessed during a mental resting state, we estimated that as few as 24 patients with Alzheimer’s disease per active treatment and placebo group would be needed to detect a significant 33% treatment response with p≤0.05 (two-tailed) and 80% power for a voxel in the left midfrontal cortex. Using the more conservative significance threshold of p≤0.005 (one-tailed), we estimated that 36 subjects would be needed per group to detect the same 33% treatment response with 80% power. These sample sizes were far smaller than the number of patients required to detect the same treatment response with the MMSE, Mattis Dementia Rating Scale, and WAIS full-scale IQ, based on data obtained in the same patients with Alzheimer’s disease followed over the 1-year interval.
The MMSE has been used widely to evaluate treatment efficacy in dementia studies
(13). Probably the most commonly used measure of general cognitive function in dementia treatment studies has been the cognitive subtest of the Alzheimer’s Disease Assessment Scale
(30). Although this cognitive test was not administered to our patients with Alzheimer’s disease, a power calculation based on a previously published value by Marin et al.
(31) for a 1-year rate of decline in 153 patients with Alzheimer’s disease and moderately severe dementia estimated that 171 patients per group would be needed to detect a similar 33% treatment response with p≤0.05 (two-tailed) and 80% power; this sample size is in the range of values found for the cognitive measures administered to our patients with Alzheimer’s disease.
In addition to findings in the left midfrontal cortex, significant declines in glucose metabolism were observed in the left inferior parietal, left midtemporal, and posterior cingulate cortices that produced sample size estimates ranging from 58 to 89 patients per group to identify the 33% treatment response with p≤0.05 (two-tailed) and 80% power. In contrast, it was estimated that using the global mean glucose metabolism value would require 179 patients per group to detect the same reduction of decline with treatment. These findings provide support for regional metabolic effects being more sensitive than global mean cerebral glucose metabolism in evaluating the progression of Alzheimer’s disease and highlight the regionally distributed heterogeneity in decline in glucose metabolism observed in Alzheimer’s disease. Thus, measurements in different brain regions may be more sensitive in detecting treatment effects over time and may be related to level of disease severity at baseline, age, or the interaction of disease severity and age.
Several PET studies have reported that cerebral hypometabolism in patients with Alzheimer’s disease is typically observed first in the parietotemporal brain regions and the posterior cingulate cortex, extending into the occipital association region and frontal cortex as the disease progresses. Our results show that the greatest decline over 1-year in patients with mildly to moderately severe dementia at baseline occurred in the frontal association regions, supporting the regional progression of Alzheimer’s disease from posterior to more anterior association brain regions over time.
A limitation of our study is the lack of healthy comparison subjects evaluated with FDG PET over the same time period as our patients with Alzheimer’s disease. Although declines in regional cerebral glucose metabolism could potentially occur in healthy aging over a 1-year interval, it is unlikely that the magnitude and extent of decline effects observed in our Alzheimer’s disease patients can be explained by aging alone. Further, the areas showing significant decline in the Alzheimer’s disease group were included in the brain regions that were hypometabolic compared with normal subjects at baseline.
Without longitudinal data for the healthy comparison subjects, we were not able to adjust the decline in regional cerebral glucose metabolism values for the effects of healthy aging in our power analyses. Thus, the treatment effects we report assume improvement to the level of no decline in the patients with Alzheimer’s disease, rather than to the level of decline that may occur in healthy aging. This may overestimate the treatment response if it is based on the assumption that the reduction of decline in patients with Alzheimer’s disease does not need to exceed the level that may be observed with aging
(32). However, since the cerebral hypometabolism of Alzheimer’s disease can begin before the onset of clinical symptoms in individuals at genetic risk for Alzheimer’s disease
(7,
33), such an assumption may not always be warranted.
We also did not make additional adjustments to the obtained sample sizes to account for potential patient attrition in treatment studies or loss of subjects attributable to poor PET data quality. These two factors could potentially increase the number of subjects needed for significant findings by 10% or 20%, depending on the type of treatment being evaluated and the PET scanning protocol used.
In a recent study by Fox et al.
(32), power calculations were performed on serially subtracted whole brain MRI scans in a group of patients with Alzheimer’s disease whose level of dementia severity was similar to that of our patients with Alzheimer’s disease. Although Fox et al. reported estimated sample sizes that were adjusted for declines in healthy aging, patient dropout, and loss of data, their unadjusted results were compatible with our findings for regional cerebral glucose metabolism with FDG PET. Unlike whole brain subtraction MRI, PET assessed during mental rest provides information on the regional distribution of disease progression and, as a measure of neuronal function, may be better able to detect the earliest effects of Alzheimer’s disease, even before the onset of clinical symptoms
(7).
Our results support the use of FDG PET during mental rest to aid in determining the longitudinal progression of pathophysiological effects of Alzheimer’s disease on a voxel-by-voxel basis and to evaluate therapies designed to slow the progression of Alzheimer’s disease over a 1-year period. Such in vivo functional brain imaging methods offer a complement to general cognitive tests in double-blind, placebo-controlled studies to evaluate treatments designed to attenuate progression of Alzheimer’s disease. Further, functional brain imaging may be useful in prevention studies for individuals at risk for dementia in the preclinical stage of Alzheimer’s disease
(7,
33).