Neuropsychological deficits are a potential part of the clinical presentation in late-life depression. Geriatric depression has been associated with impairment in short-term memory
(1), visuospatial skills
(2,
3), and psychomotor functioning
(1). Studies of geriatric patients with major depression have documented disturbances in executive functioning
(3–
5), including impaired planning, organizing, initiating, sequencing, shifting, information processing speed, and working memory.
A review of the literature revealed inconsistent findings regarding executive deficits in depression and suggests that frontal impairment varies according to age and depression severity
(6). Severely depressed older adults have been found to evidence impairment in set shifting, verbal fluency, psychomotor speed, recognition memory, and planning on the Cambridge Neuropsychological Test Automated Battery
(7). In another study using the same battery
(8), moderately depressed middle-aged patients demonstrated deficits in planning, strategy development, spatial working memory, and verbal fluency despite exhibiting intact set shifting ability and psychomotor speed. In yet another study
(9), severe depression was associated with deficits in set shifting but intact verbal fluency. Despite some equivocal findings in the literature, it is generally accepted that while performance on tasks requiring development of performance strategies may suffer in depressed patients, automatic processes are relatively preserved
(10).
Although focal deficits or even severe global impairment are observed in some depressed elderly patients, the cognitive functioning of others remains intact. This variation may result from the biologic heterogeneity of depression. Studies of the cognitive response to psychopharmacological treatment of late-life depression indicate that a substantial number of patients continue to experience residual signs of the disorder, including neuropsychological deficits. Persisting mild memory and executive impairment have been observed in elderly depressed patients who achieved remission with antidepressant treatment
(4).
Recently, one of us
(11) proposed that depressive symptoms and executive impairment originate from related brain dysfunction, at least in a subgroup of elderly patients. The clinical presentation of elderly patients with the “depression–executive dysfunction syndrome” is characterized by psychomotor retardation and reduced interest in activities but a less pronounced vegetative syndrome than is seen in depressed elderly patients without significant executive dysfunction
(12). The neuropsychological dysfunction of patients with depression–executive dysfunction syndrome consists of impaired verbal fluency and visual naming and poor performance on tasks of initiation and perseveration
(12). Depressive ideation, psychomotor retardation, and executive dysfunction in depressed elderly patients are associated with compromised instrumental activities of daily living
(13,
14).
Several symptoms of depression–executive dysfunction syndrome, namely psychomotor retardation, apathy, and fluency deficits, resemble impairments exhibited in frontal lobe syndromes. However, in contrast to patients with frontal lobe syndromes, patients with depression–executive dysfunction syndrome meet criteria for major depression and have depressive symptoms comparable in severity to those of geriatric patients with major depression who have unimpaired executive functions
(15). Delayed or poor
(16) and unstable
(15) responses to antidepressants have been documented in elderly patients with major depression and executive dysfunction. In contrast, memory impairment does not predict antidepressant response, relapse, or recurrence of late-life depression
(15).
Neuroimaging studies of major depression have identified neuroanatomical and neurophysiological abnormalities most consistently in the frontal lobes. Magnetic resonance imaging of depressed patients has revealed structural abnormalities in areas related to the cortical-striatal-pallidal-thalamus-cortical pathways
(17), including the frontal lobes
(18), caudate
(19), and putamen
(20). Positron emission tomography has shown hypometabolism in a variety of areas that mediate executive functions, including the dorsolateral prefrontal cortex
(21–
23), anterior cingulate
(24,
25), and basal ganglia
(26). Hypometabolism of the rostral anterior cingulate was found to be associated with resistance to antidepressants in younger depressed patients
(25). Notably, however, an increase in frontal hypometabolism has been reported after successful treatment with antidepressant medications
(27,
28).
Subtle executive dysfunction occurs with normal aging
(29), prompting questions about the concomitant effects of aging and depression on neuropsychological functioning. Some have argued that poor neuropsychological functioning may result from the combined effects of aging and depression. Raskin and colleagues
(30,
31) maintain that an interaction between aging and depression minimizes cognitive flexibility and compromises processing speed. Neurobiological models of aging and of depression that have common neurophysiologic and neuroanatomic substrates have been proposed
(32). Accordingly, delineation of neuropsychological functioning in late-life depression is both theoretically and clinically important.
The purpose of the present study was to characterize the neuropsychological presentation of geriatric depression with a particular focus on attention and executive functions. On the basis of a neuropsychological approach
(33,
34), four neurocognitive domains were studied: two involving attentional processes (selective attention and sustained attention) and two involving higher-order executive functions (inhibitory control and focused effort). Selective attention requires perceptual sensitivity to signal/noise differences, reflecting the ability to discriminate target items from distractor items. Sustained attention refers to the temporal consistency in performance, specifically the ability to persist over time as a function of overall productivity. Inhibitory control involves initiation, active switching, and inhibition of overlearned responses. Finally, focused effort refers to the intensity of directed attention and involves working memory, speed of processing, and complex mental operations. Selective and sustained attention tasks involve orienting responses and execution of learned sequences. In contrast, inhibitory control and focused effort involve novel situations that necessitate planning, error correction, suppression of temptation, or spontaneous development of performance strategies.
It was hypothesized that depressed subjects, regardless of age, would demonstrate poor performance on attention tasks and on tasks purportedly subserved by the frontal lobes (i.e., tasks requiring response initiation, behavioral inhibition, active set shifting, and quick processing speed). It was further hypothesized that older depressed adults would have a significantly greater degree of impairment than their younger depressed counterparts on these tasks, while no interaction between age and depression was anticipated for the tasks of selective or sustained attention.
Method
Study Group
Young and elderly adults with major depressive disorder (N=40) were consecutively recruited for participation through the Cornell Institute for Geriatric Psychiatry. Young and elderly healthy comparison subjects (N=40) with no psychiatric history were recruited from the community. Upon recruitment, all study candidates were assessed with the Mini-Mental State Examination (MMSE)
(35) and the Schedule for Affective Disorders and Schizophrenia—Lifetime Version (SADS-L)
(36) by an experienced clinician. The exclusion criteria included 1) significant medical illness (i.e., history of metastatic cancer, brain tumor, myocardial infarction, or stroke), 2) history of head trauma or epilepsy, 3) delirium, dementia, Huntington’s chorea, or Parkinson’s disease, 4) history of substance abuse, 5) hearing, vision, or motor impairment that precluded neuropsychological testing, and 6) an MMSE score lower than 24. All of the depressed subjects met the Research Diagnostic Criteria
(37) for a current diagnosis of major depressive disorder without psychotic features. Depressed patients with a history of manic episodes, treatment with ECT, or comorbid psychiatric disorder were excluded. All subjects signed informed consent statements.
The subjects were grouped by diagnosis (depressed versus comparison) and by current age (young versus old; young=20–60 years, old=61 years and above). The four subject groups (young comparison, young depressed, old comparison, old depressed) were composed of 20 subjects each.
Instruments
All subjects were evaluated by means of a test battery comprising the following neuropsychological instruments: California Verbal Learning Test
(38), Conners’ Computerized Continuous Performance Test
(39), category fluency test
(40, pp. 447–464), digit span and digit symbol subtests of the Wechsler Adult Intelligence Scale, 3rd edition (WAIS-III)
(41), finger tapping test
(42, pp. 229–234), Stroop Color and Word Test
(43), Trail Making Test
(42, pp. 279–288), Visual Cancellation Test
(44), Auditory Cancellation Test
(44;
45, pp. 548–549), and Wisconsin Card Sorting Test
(46). Task variables selected from these instruments were grouped to render indices of performance on four neuropsychological domains: selective attention, sustained attention, inhibitory control, and focused effort (
Appendix 1).
Domain 1: selective attention
Selective attention was evaluated with d prime (d′) indices derived from five tests: a) the Continuous Performance Test—a fixed-interval computerized test of vigilance requiring the subject to quickly press a button in response to targets while refraining from responding to nontargets; b) the Visual Cancellation Test—a paper-and-pencil measure of visual scanning requiring cancellation of targets intermittently placed in a large array; c) the Auditory Cancellation Test—an auditory adaptation of visual cancellation requiring the subject to raise his or her hand in response to targets while listening to a standardized audiotape during which letters are read aloud; d) the Stroop Color and Word Test—a task requiring rapid naming of the color of ink in which words representing incongruent colors are printed; and e) the California Verbal Learning Test—a test requiring recall and recognition of items from an orally presented shopping list.
The d′ variables for these tests served as indices of perceptual sensitivity, that is, the ability to discriminate target items from distractor items. All measures of d′ were calculated by using the following formula adapted from signal detection theory
(47): Zc–Zh (where Zc represents the normal curve deviate for the proportion of commission errors and Zh represents the normal curve deviate for the proportion of hits). Statistically, this sensitivity measure represents the distance along the x axis between the noise distribution and the signal distribution, in standard-score units.
Domain 2: sustained attention
Sustained attention was assessed by using decrement scores derived from four tests: a) the WAIS-III digit symbol subtest—a test requiring rapid copying of symbols paired with numbers; b) category fluency test—a test requiring rapid generation of animal names in 60 seconds; c) sustained finger tapping—tapping with the index finger of the dominant hand over 60 seconds; and d) the color-word task from the Stroop Color and Word Test. Data for these measures were recorded in blocks to provide an index of the temporal consistency in performance. Decrement scores were determined by calculating the discrepancy in productivity between the first and last halves of the test as a function of total productivity. For example, for the category fluency test, the number of words generated during the last half of the test was subtracted from word generation during the first half, and the difference was divided by the total number of words produced. This approach permitted investigation of attention functioning over time while avoiding erroneous generalizations about decrement in performance for the subjects with minimal overall productivity. In addition to these decrement scores, sustained attention was assessed by using a reaction time block change score from the Continuous Performance Test (slope of change in reaction times over the six time blocks).
Domain 3: inhibitory control
Measures of active set shifting and perseveration were used to assess inhibitory control. These included a) commission errors on the Continuous Performance Test—an index of nontarget responses on the computerized vigilance test; b) perseverative errors on the Wisconsin Card Sorting Test—a test requiring the subject to sort cards with no explanation of the sorting principles; c) perseverative errors on the California Verbal Learning Test; d) perseverative errors on the category fluency test; and e) completion time for Trail Making Test part B—a paper-and-pencil test of alphanumeric sequencing.
Domain 4: focused effort
The focused effort domain was assessed by way of tasks requiring effortful processing, mental manipulation, or quick processing speed. These included a) number of items completed for the WAIS-III digit symbol subtest; b) items completed for the WAIS-III digits backward subtest—a task requiring verbal recall of digit strings in a backward sequence; c) total words produced for category fluency; d) items completed on the color-word trial of the Stroop Color and Word Test; and e) Continuous Performance Test reaction time—the mean response time in milliseconds for all target responses over the six time blocks.
Data Analysis
The performance ratings of the four subject groups on each of the four neuropsychological domains were compared by using multivariate analyses of variance (MANOVAs). Univariate analyses with pairwise comparisons were then performed to examine specific group differences. For the omnibus tests, alpha was set at 0.05. For the pairwise comparisons, the alpha level was adjusted by using Bonferroni correction (p<0.01).
Results
Demographic and Clinical Characteristics
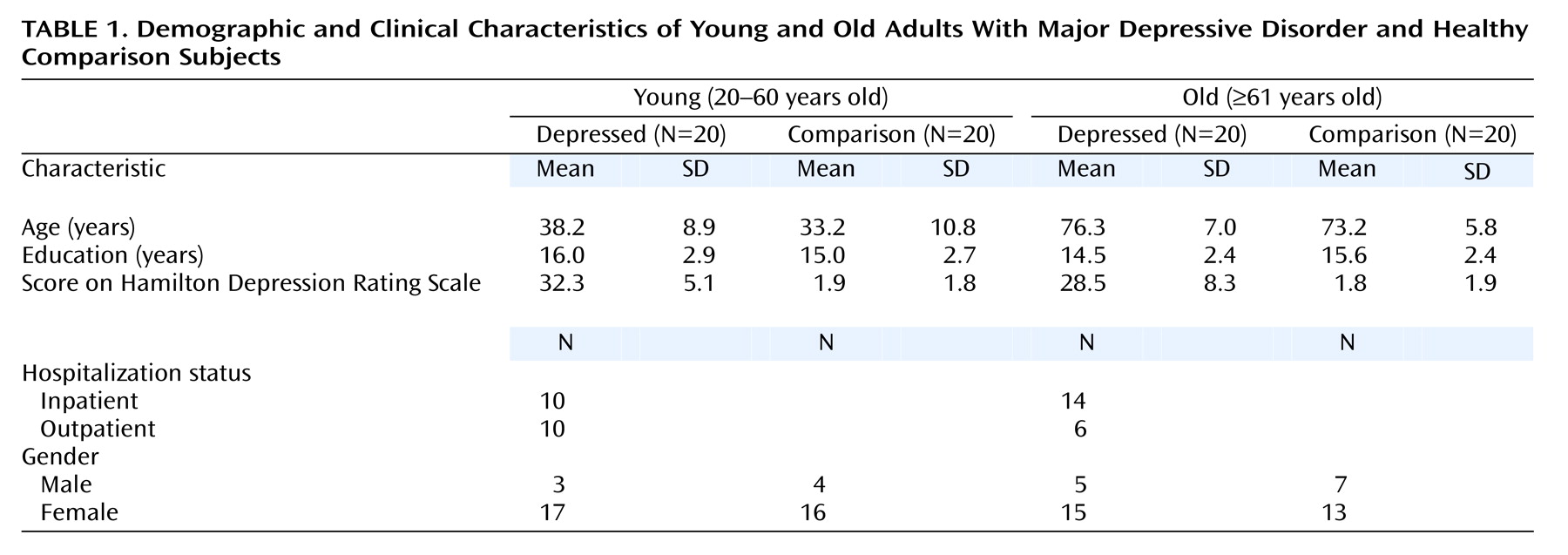
The overall study group comprised 19 men (23.8%) and 61 women (76.3%); their mean age was 55.1 years (SD=21.3, range=20–87), and their mean education level was 15.3 years (SD=2.6, range=12–23). The subjects were predominantly Caucasian but included three African Americans. Depression severity was assessed with the Hamilton Depression Rating Scale
(48), which revealed that as a whole, the depressed patients were severely depressed (mean score=30.38, SD=7.17). Of the depressed patients, 21 were taking psychotropic medications at the time of testing: 14 were taking an antidepressant only (eight young, six old), two were taking a benzodiazepine only (one young, one old), and five were taking an antidepressant coupled with a benzodiazepine (four young, one old).
Table 1 lists the demographic and clinical characteristics of the four subject groups.
As shown in
Table 1, the young comparison and young depressed subjects were well matched for age (t=–1.62, df=38, p=0.11), as were the old comparison and old depressed subjects (t=–1.53, df=38, p=0.14). There were no significant differences between the depressed and comparison groups in years of education (t=0.04, df=78, p=0.97). Nor did a one-way analysis of variance of education level indicate significant differences among all four subject groups (F=1.21, df=3, 76, p=0.32). No significant difference in depression severity, as assessed by the Hamilton depression scale, was found between the two depressed subgroups (t=1.69, df=38, p=0.10).
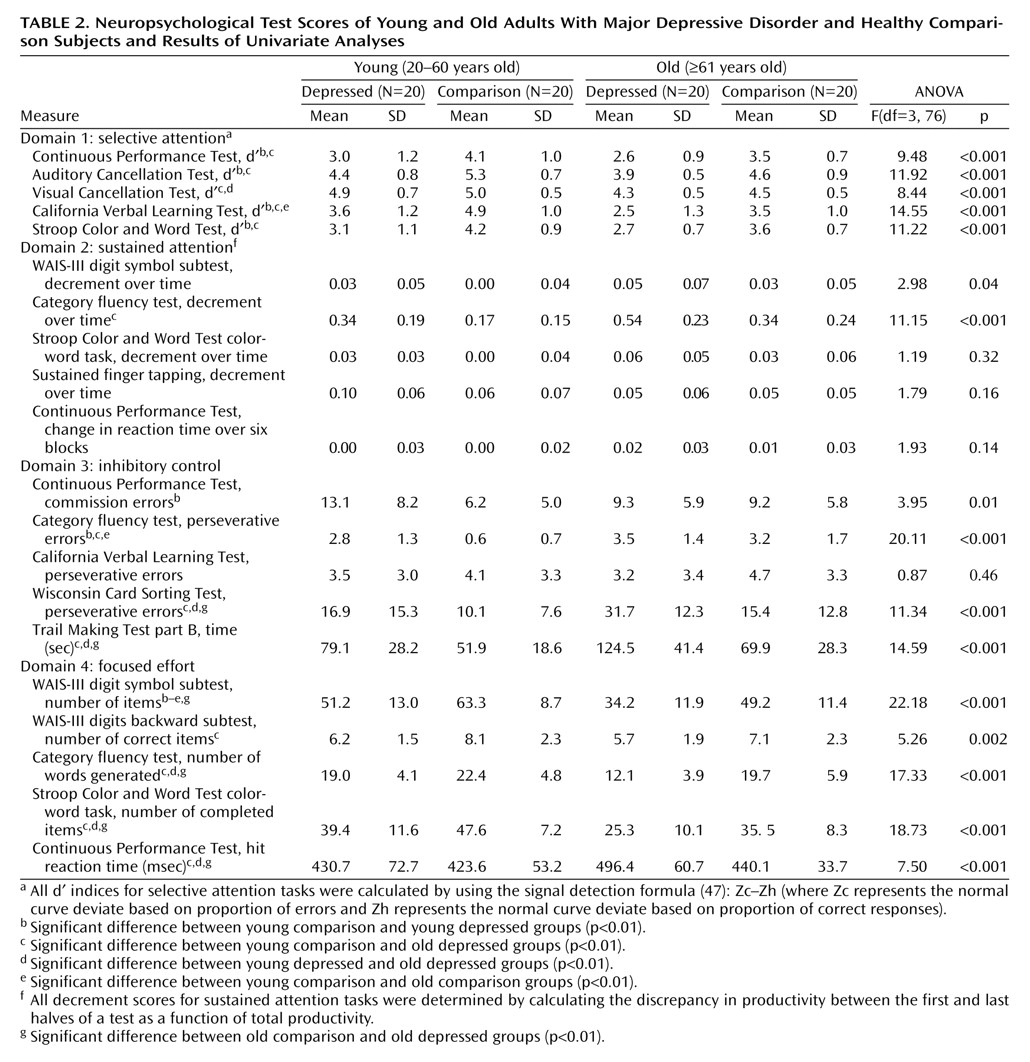
Table 2 lists the neuropsychological scores of the four subject groups.
Results by Neuropsychological Domain
Domain 1: Selective Attention
MANOVA revealed no significant interaction between age and depressive state for the selective attention domain (Wilks’s lambda=0.98, F=0.32, df=5, 72, p=0.90). Relative to the comparison subjects, however, the depressed patients performed more poorly on this domain (Wilks’s lambda=0.61, F=9.40, df=5, 72, p<0.001). The depressed patients exhibited greater difficulty discriminating targets from nontargets in signal detection tasks (Auditory Cancellation Test d′, Continuous Performance Test d′, California Verbal Learning Test d′). As a group, older subjects performed more poorly than younger subjects on selective attention tasks (Wilks’s lambda=0.59, F=10.18, df=5, 72, p<0.001). They exhibited a deficit in the ability to appropriately select the relevant stimulus dimension (Stroop Color and Word Test d′) and less accurate differentiation of target and distractor stimuli (Visual Cancellation Test d′, Auditory Cancellation Test d′, California Verbal Learning Test d′). However, when the comparisons were restricted to the healthy subjects, the older subjects showed no significant deficits in selective attention.
Domain 2: Sustained Attention
In keeping with the results from the selective attention tests, MANOVA revealed no significant age-depression interaction associated with the sustained attention domain (Wilks’s lambda=0.93, F=1.67, df=5, 72, p=0.33). The depressed patients performed significantly worse than the comparison subjects on this domain (Wilks’s lambda=0.74, F=4.95, df=5, 72, p=0.001) but showed a more precipitous decline in performance from the initial to the latter stages for only a single test (category fluency). A significant age effect was found for the sustained attention domain (Wilks’s lambda=0.72, F=5.57, df=5, 72, p<0.001), although only on the category fluency task did the elderly patients as a group evidence significantly greater difficulty maintaining performance over the course of the task. Further, the sustained attention of the healthy older adults was comparable to that of their younger healthy counterparts.
Domain 3: Inhibitory Control
As hypothesized, MANOVA revealed a significant age-depression interaction for the inhibitory control domain (Wilks’s lambda=0.74, F=5.05, df=5, 72, p=0.001). The elderly depressed adults demonstrated the most difficulty with active set shifting, as evidenced by disproportionately poor scores on Trail Making Test part B. They also showed the greatest tendency to produce perseverative responses on tasks of conceptualization (Wisconsin Card Sorting Test) and initiation (category fluency). The young depressed patients produced the highest number of commission errors on the Continuous Performance Test, and the depressed group as a whole produced a significantly higher number of commission errors than the comparison subjects.
Domain 4: Focused Effort
In keeping with the results from the inhibitory control tasks, MANOVA for the focused effort domain revealed a significant age-depression interaction (Wilks’s lambda=0.85, F=2.57, df=5, 72, p=0.03). The elderly depressed patients demonstrated the longest response latencies on the Continuous Performance Test and copied the fewest symbols on the WAIS-III digit symbol subtest, suggesting compromised processing speed. They also demonstrated the weakest capacity for initiation on the category fluency task and the slowest processing time on the color-word trial of the Stroop Color and Word Test (as indicated by fewer completed items).
Discussion
The principal finding of this study is that geriatric depression is characterized by impaired executive functioning. Relative to their depressed younger counterparts and to the healthy elderly cohort, depressed geriatric subjects had disproportionately poor scores on neuropsychological tasks associated with frontal lobe integrity. Dissection of the neuropsychological impairment in the current group of depressed geriatric patients reveals primary impairment of response initiation and inhibition, active switching, processing speed, and complex mental manipulation. While the stability over time of these deficits requires further investigation, the results of this study are in keeping with previous descriptions
(49,
50) of an association between late-life depression and executive deficits.
With respect to the effects of depression, the total depressed group, as hypothesized, evidenced mild weaknesses in perceptual sensitivity and isolated difficulties in maintaining their level of performance over time. These results are in keeping with reports of deficits in both selective attention
(51) and sustained attention
(52) for depressed patients and suggest that depression may contribute to susceptibility to interference, with a resultant compromise in the ability to filter noise and attend to signals. In the present study, depressed patients also had a greater tendency to perseverate and committed significantly more Continuous Performance Test commission errors than healthy comparison subjects. These findings support previous work documenting difficulties in set shifting in depression
(7) and impairments on tests requiring inhibition of responses to distractor stimuli, such as the Continuous Performance Test
(53,
54).
With respect to age effects, the healthy older adults had no more difficulty discriminating relevant and distractor stimuli than did the young healthy adults, nor was older age in healthy adults associated with poor ability to maintain performance over the course of tasks. Similar reports indicating intact selective attention for healthy older adults are found in the literature
(55), as are reports of a lack of fatigue effects during neuropsychological tasks
(56). In contrast, executive dysfunction in set formation and set shifting (for both verbal and nonverbal domains) and slowed psychomotor speed have been documented for healthy older adults
(29). The results from the current study reflect similar findings: the older healthy adults demonstrated deficits in set shifting and slower psychomotor performance relative to their healthy young counterparts.
The potential contribution of age effects to neurocognitive deficits found in this study must be considered, particularly since a frontal lobe hypothesis of age-related cognitive changes has been proposed
(57). Our findings support such a hypothesis, given that relative to healthy young adults, the only deficits exhibited by the healthy older adults were in executive domains. Most critical to the study objectives, however, is the finding that executive deficits were associated with
both older age and depression and that an interaction between these variables was documented for both neuropsychological domains of executive function. The results suggest deficits in processing resources for both depressed patients and older adults, with these impairments compounded for depressed geriatric patients. These deficits are most evident on tasks requiring set shifting, inhibition of prepotent responses, spontaneous use of learning strategies, and quick psychomotor speed.
The exact mechanisms underlying executive dysfunction in geriatric depression remain to be identified. A potential mechanism may be mediated by the cortical-striatal-pallidal-thalamus-cortical pathways, as these circuits are strongly implicated in the development of spontaneous performance strategies demanded by executive tasks. In particular, the dorsolateral prefrontal cortex mediates planning and response sequencing
(58), as well as focusing and active switching
(33), while the orbitofrontal pathway subserves response initiation and inhibition
(59). Response intention and focusing are also influenced by the cingulate cortex
(60). Notably, abnormalities (of both cerebral blood flow and glucose metabolism) in these areas have been consistently documented in positron emission tomographic studies of patients with major depression. In a recent review of functional neuroimaging studies, Liotti and Mayberg
(61) concluded that a majority of findings implicate the right dorsolateral prefrontal cortex as a primary site of functional abnormalities associated with poor externally directed attention in major depression. They further contended that the anterior cingulate cortex may be the site of metabolic abnormalities associated with impaired ability to inhibit inappropriate responses in depressed individuals. Compounding the executive dysfunction in
older depressed adults may be age-related hypometabolism
(62) and volumetric reductions in prefrontal and orbital-frontal areas
(63).
The clinical significance of this study is that the delineation of specific executive and attention deficits in depressed elderly patients may facilitate the development of effective treatment interventions. Timely identification of attentional and executive processes fundamental to the daily functioning of depressed older adults may lead to compensatory strategies that will improve the outcomes of late-life depression. Such interventions are much needed in these patients, because at least some of them may be resistant to pharmacotherapy
(15,
16).
The heuristic significance of this study is that it outlines the cognitive functions most affected in geriatric depression and may be used for the development of models for understanding the role of age-related changes in late-life depression. If specific executive dysfunctions are attributable to impairment of cortical-striatal-pallidal-thalamus-cortical systems, at least two models can be proposed. In the first model, depression is viewed as a condition unmasking executive dysfunctions in patients with compromised cortical-striatal-pallidal-thalamus-cortical systems. In this model no pathophysiological relationship between dysfunction of these systems and the mechanisms of depression is assumed. In the second model, dysfunction of cortical-striatal-pallidal-thalamus-cortical pathways is conceptualized as mediating or predisposing to both depression and specific executive dysfunction (i.e., depression and executive dysfunctions are not independent). This assertion is supported by studies suggesting that the course of geriatric depression is influenced by some executive dysfunctions
(49,
50). The theoretical advantage of the second model is that it can guide neuroimaging studies to investigate the functional neuroanatomy of cortical-striatal-pallidal-thalamus-cortical circuitry and its role in geriatric depression. Tasks of inhibitory control and focused effort may be modified and used as stimuli in neuroimaging studies, as these processes were found to be abnormal in this study.
This study addressed some of the limitations of previous investigations by using rigorous methodological constraints, including use of well-matched healthy comparison groups and stringent exclusion criteria. Assessment with a state measure (Hamilton depression scale) assured inclusion of depressed subjects with similar levels of current depression severity. Controlling for education and health status helped to prevent introduction of variance that might obscure group differences. Use of a comprehensive neuropsychological battery lent additional rigor by not only allowing for assessment of specific aspects of attention and executive functions, but also by tapping multiple performance modalities (verbal, visual, motor).
This study is limited by its cross-sectional design with discrete age groupings, a feature that precludes conclusions about the timing of age effects in patients with major depression. Ideally, a study of this nature would have examined age as a continuous variable. The small number of subjects prohibited such a design. Future studies should address the issue of whether age-related declines in depressed and nondepressed older adults are similar in their onset. The focus on subjects with severe depression led to the inclusion of some subjects who were receiving medication, although there were no significant differences in the mean number of young and old medicated patients in this study. While we cannot exclude an effect of medications on test performance, we believe that this concern is mitigated by the fact that additional analyses did not demonstrate differences between patient groups based on medication status for any of the four neuropsychological domains. Further, selective serotonin reuptake inhibitors and venlafaxine, which were the antidepressants used by most of our subjects, do not have a strong effect on neuropsychological test performance.
In conclusion, the theoretical contribution of this study is the delineation of attentional and executive processes in depressed older patients, which can be used to devise stimulated functional neuroimaging studies that would clarify the pathophysiology of geriatric depression. Findings from this study may be used clinically to guide the design of focused interventions. Specific cognitive retraining and/or remediation, as well as assistance with disability resulting from executive dysfunction, may prove to be effective therapeutic additions to the current treatment armamentarium for geriatric depression, which leaves many patients symptomatic and suffering.