Magnetic resonance imaging (MRI) studies support the presence of superior
(1) and medial
(2,
3) temporal lobe abnormalities in psychotic illness. Although studies of chronically ill patients are susceptible to the confounding effects of long-term neuroleptic exposure and neurodegenerative change
(1,
3,
4), patients experiencing their first psychotic episode demonstrate similar abnormalities
(3–
6), suggesting that the abnormalities are not simply an effect of chronicity. However, studies of both chronically ill patients and those experiencing their first psychotic episode are susceptible to more general confounds of gender, handedness, and diagnostic heterogeneity
(2,
7). These confounds may be linked to inconsistent results in the literature on patients experiencing their first psychotic episode
(7–
9), which have left the nature and time course of temporal lobe changes in schizophrenia unclear.
In this study we measured the temporal lobe structures of minimally treated, right-handed male patients who were scanned early during their first psychotic episode as well as in comparison subjects matched for gender and handedness. In this tightly controlled study group, we predicted that patients would have smaller hippocampal and superior temporal gyrus volumes than healthy subjects.
Method
Recruitment, exclusion, and demographic assessment details have been described elsewhere
(10). Briefly, 25 minimally medicated (cumulative exposure=less than 12 weeks), right-handed men experiencing their first psychotic episode and 16 healthy, right-handed male comparison subjects participated. The patients’ mean age was 24.0 years (SD=4.7); their mean height was 173 cm (SD=9.0); their mean socioeconomic status was 2.8 (SD=0.7); their mean number of years of education was 12.0 (SD=1.5); their mean age at onset of first psychotic symptoms, based on patient interview, was 24.0 years (SD=5.0); their mean illness duration (age at onset minus age at MRI scan) was 8.0 months (SD=7.6); and their mean neuroleptic exposure was 3.0 weeks (SD=3.2). The mean age of the comparison subjects was 27.0 years (SD=5.9); their mean height was 176.0 cm (SD=9.0); their mean socioeconomic status was 2.6 (SD=0.9); and their mean number of years of education was 15.0 (SD=2.5).
Diagnosis was determined by administration of the Structured Clinical Interview for DSM-III-R—Patient Version 1.0
(11) by qualified research psychiatrists (D.G.F., S.O., or V.C.D.). Twelve patients met criteria for schizophrenia (paranoid N=7, disorganized N=3, undifferentiated N=2), 10 for schizophreniform psychosis, and three for schizoaffective disorder. Comparison subjects with a personal history of any axis I or II disorder or a family history of psychosis were excluded. After a full description of the study, all subjects provided written informed consent.
T1-weighted MRI scans were acquired by using a 1.5-T scanner (General Electric Medical Systems, Milwaukee) with the following sequence: TR=11.3 msec, TI=300 msec, TE=2.2 msec, flip angle=20° EX=1), producing 124 1.5-mm thick axial slices covering the entire brain.
Volumetric measurements were obtained by means of stereological assessment
(10). Three of us (N.M., A.F., and A.S.) measured hippocampal, amygdala, Heschl’s gyrus, and planum temporale volumes blind to diagnosis. Hippocampal, Heschl’s gyrus, and planum temporale definitions were based on previously published criteria
(3,
12). The amygdala was defined superiorly by the caudate head, medially by the uncus, and inferiorly by the entorhinal cortex. Interrater reliability and intrarater reliability (over a period of 4 months) were assessed on 10 scans. Intraclass correlations ranged from 0.90 to 0.95.
We used chi-square analyses (SPSS-10.0, SPSS, Inc., Chicago) to test for differences in socioeconomic status and ethnicity; we used t tests to evaluate differences in years of education, age, and height. We used multivariate analysis of covariance (MANCOVA) to compare group (patients versus comparison subjects) differences in all regions of interest, covarying for number of years of education and height. Height correlates highly with head size and has previously been used in brain structure studies
(3,
10)). Since the amygdala has been implicated in paranoid psychosis
(13), an exploratory MANCOVA was performed to test for amygdala volume differences between patients with paranoid psychosis (N=7) and those with nonparanoid psychosis (N=8). This analysis excluded patients with schizophreniform disorder because they had the potential to develop paranoid psychosis and to have different illness durations. Covariates for this analysis were education, height, age at onset of illness, and illness duration.
Results
Comparison subjects had completed more years of formal education than patients (t=5.7, df=39, p<0.001), and patients with nonparanoid psychosis had completed more years than those with paranoid psychosis (t=3.5, df=13, p<0.004). Groups with paranoid (mean=164.3, SD=7.9) and nonparanoid (mean=175.0, SD=5.3) psychosis differed significantly in height (t=3.1, df=13, p<0.008). There were no other demographic differences.
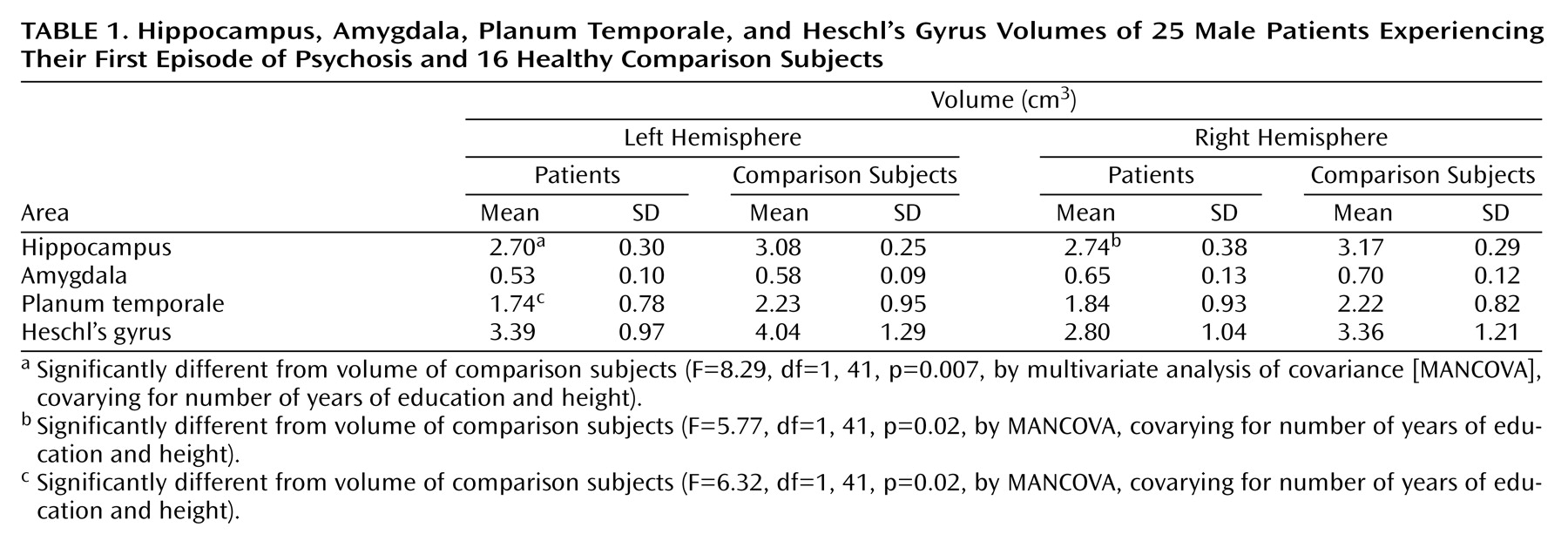
Table 1 displays the regional volumes of patients and comparison subjects. MANCOVA showed a significant effect for group (F=2.64, df=8, 30, p=0.03). Patients had smaller hippocampal and left planum temporale volumes than comparison subjects on the right and the left. No significant main effect was found between patients with paranoid and nonparanoid psychosis (F=2.30, df=2, 7, p=0.17). Patients with paranoid psychosis had smaller left amygdala volumes than the patients with nonparanoid psychosis (F=5.19, df=1, 15, p=0.05).
Discussion
Assessment of temporal lobe substructures with careful controls for potential confounds demonstrated smaller bilateral hippocampal and left planum temporale volumes in male patients experiencing their first episode of psychosis. This finding is consistent with those of previous studies
(3,
5).
Other studies suggested that smaller volume of the left hippocampus is more predominant in patients experiencing their first psychotic episode
(9) and that abnormalities in the right hippocampus may be more dependent on illness progression
(3) (but see reference
2). Reduction of the superior temporal gyrus volume is also found to progress with illness duration
(1,
4). This may explain why we did not find any reduction in Heschl’s gyrus volume, which has been found by others
(5). Further longitudinal studies, beginning at first-episode or prodromal stages, are required to study differential effects of illness duration on these structures.
Although the small number of patients with paranoid psychosis in our study necessitates caution in interpretation of the findings, these patients had smaller left amygdala volumes than patients with nonparanoid psychosis. Previous studies also found differences in the left amygdala between patients with paranoid and nonparanoid illness
(13). Research with larger numbers of subjects should explore this further. Such differences would pose an additional confound and should be controlled for in future studies.
In conclusion, our results suggest that bilateral hippocampal and left planum temporale volumes are smaller in patients experiencing their first psychotic episode than in healthy comparison subjects. Differences in amygdala volumes may differentiate diagnostic subtypes.