Major depression has been the most studied psychiatric disorder after traumatic brain injury. The rates of axis I disorders in patients with traumatic brain injury are 14%–77% for major depression
(1,
3–
5,
8–10), 2%–14% for dysthymia
(1,
4,
5,
9), 2%–17% for bipolar disorder
(3,
7–9), 3%–28% for generalized anxiety disorder
(1,
6,
8–10), 4%–17% for panic disorder
(1,
8–10), 1%–10% for phobic disorders
(8–
10), 2%–15% for obsessive-compulsive disorder
(8–
10), 3%–27% for posttraumatic stress disorder (PTSD)
(9,
10,
12), 5%–28% for substance abuse or dependence
(1,
8–10), and 1% for schizophrenia
(3,
10).
Since the famous case of Phineas Gage in 1848
(13), personality change has been reported in 49% to 80% of patients with traumatic brain injury
(14–
16). Franulic et al.
(17) found ICD-10 organic personality disorder in 32% of patients after traumatic brain injury. To our knowledge, there have been only two studies that used structured interviews and diagnostic criteria to examine the occurrence of all personality disorders after traumatic brain injury. Van Reekum et al.
(8) found DSM-III-R personality disorders in seven (39%) of 18 patients. Hibbard et al.
(11) investigated 100 individuals for DSM-IV personality disorders an average of 8 years after traumatic brain injury. Sixty-six percent of the patients had at least one personality disorder, and the most common were borderline (34%), obsessive-compulsive (27%), paranoid (26%), avoidant (26%), and antisocial (21%).
The aim of this study was to evaluate the occurrence of axis I and II disorders after traumatic brain injury. The average follow-up of the patients was 30 years, which is, to our knowledge, the longest ever reported.
Method
This study was a retrospective follow-up. The subjects were recruited from a group of 210 patients who had received traumatic brain injuries between 1950 and 1971 and who had been referred for neuropsychological evaluation to one of us (R.P.) at Turku University Central Hospital (Turku, Finland) between 1966 and 1972. The reason for the referral was either a recent injury or significant disability after an earlier injury. At that time the diagnosis of traumatic brain injury was made on the basis of neurological symptoms and their consistency with the type of injury, whereas neuroradiological examinations were seldom carried out.
From the original group of 210 patients, 76 had died. The inclusion criteria for the remaining 134 patients were 1) a head trauma severe enough to cause traumatic brain injury and causing neurological symptoms (including headache and nausea) lasting at least 1 week and 2) at least one of the following: loss of consciousness for at least 1 minute, posttraumatic amnesia for at least 30 minutes, neurological symptoms (excluding headache and nausea) during the first 3 days after the injury, or neuroradiological findings suggesting traumatic brain injury (e.g., skull fracture, intracerebral hemorrhage). The exclusion criteria were 1) neurological illness before the brain injury, 2) clinical symptoms of a nontraumatic neurological illness that developed after the traumatic brain injury (excluding dementia), 3) insufficient cooperation, or 4) unavailability of medical records.
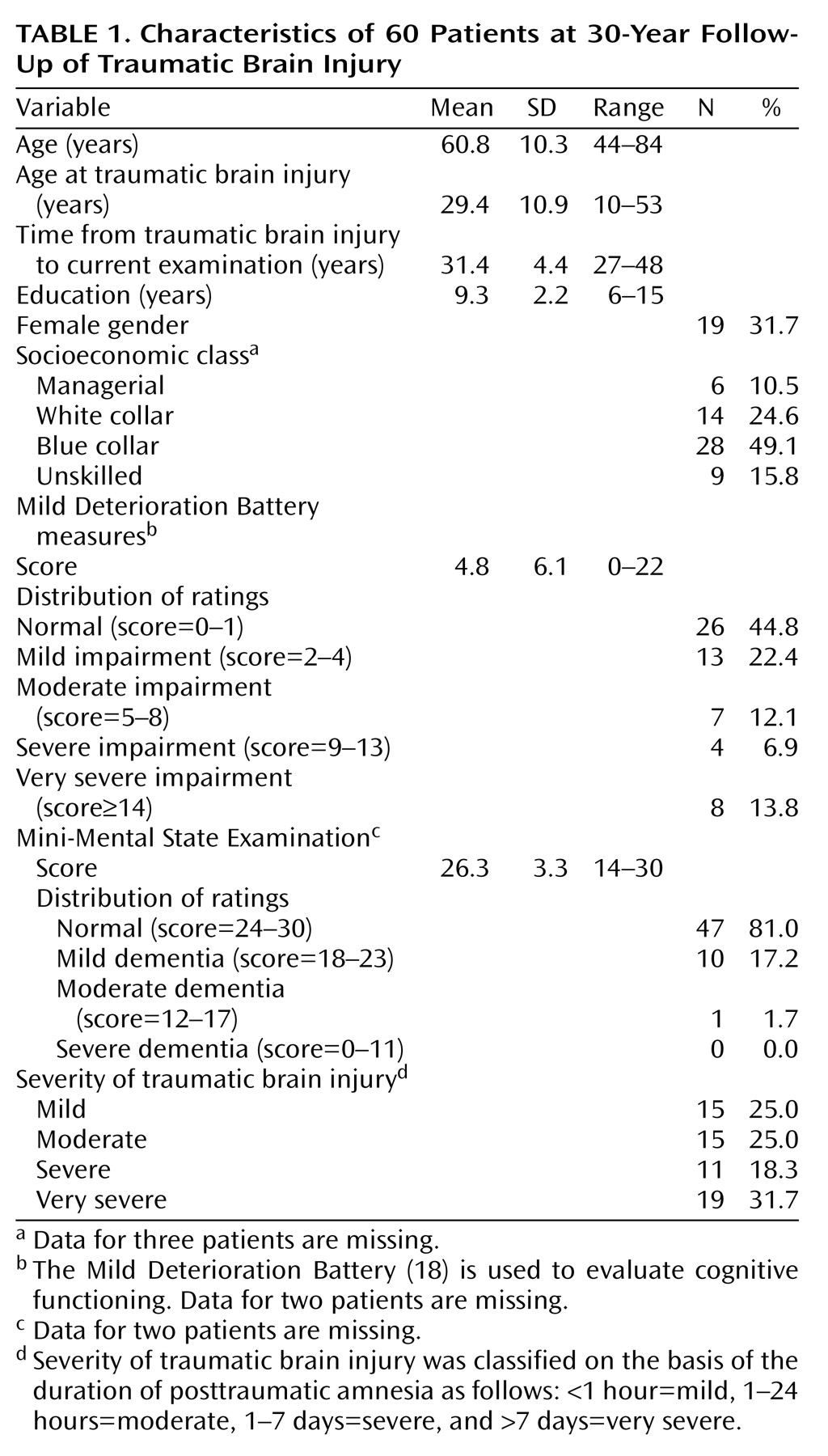
Of the 134 patients, 13 did not meet the inclusion criteria according to medical records, one patient was excluded because of neurological illness before traumatic brain injury, and two patients did not have available medical records. The remaining 118 patients were contacted by mail, and 88 of them replied. Eighty-three of them met the inclusion criteria, but seven were excluded because of a nontraumatic neurological illness, and 16 refused to participate in the study. The remaining 60 patients formed the study group for this investigation, and they were examined between January 1998 and April 1999. After complete description of the study to the subjects, written informed consent was obtained. The protocol was approved by the Conjoint Ethics Committee of Turku University and Turku University Central Hospital. The characteristics of the patients are presented in
Table 1.
To test the representativeness of the study group (N=60), the deceased subjects (N=76) plus the combined group of subjects (N=46) who either refused to participate in the study (N=16) or could not be reached (N=30) were compared with the study group in terms of age, gender, education, severity of traumatic brain injury, and history of harmful alcohol use dichotomized as yes or no (data were missing for 6.7% of the study group, 27.6% of those who were deceased, and 45.7% of those who refused to participate or could not be reached). The only significant differences between the groups, according to analysis of variance (ANOVA), were in age (F=29.42, df=2, 179, p<0.001) and education (F=9.77, df=2, 179, p<0.001). The deceased subjects were significantly older and had less education.
Background data were collected with a specially designed questionnaire containing information on demographic characteristics and traumatic brain injury. The severity of traumatic brain injury was classified on the basis of the duration of posttraumatic amnesia as follows: <1 hour=mild, 1–24 hours=moderate, 1–7 days=severe, and >7 days=very severe. There was no significant difference in the severity of traumatic brain injury between men and women (Fisher’s exact test, p=0.24).
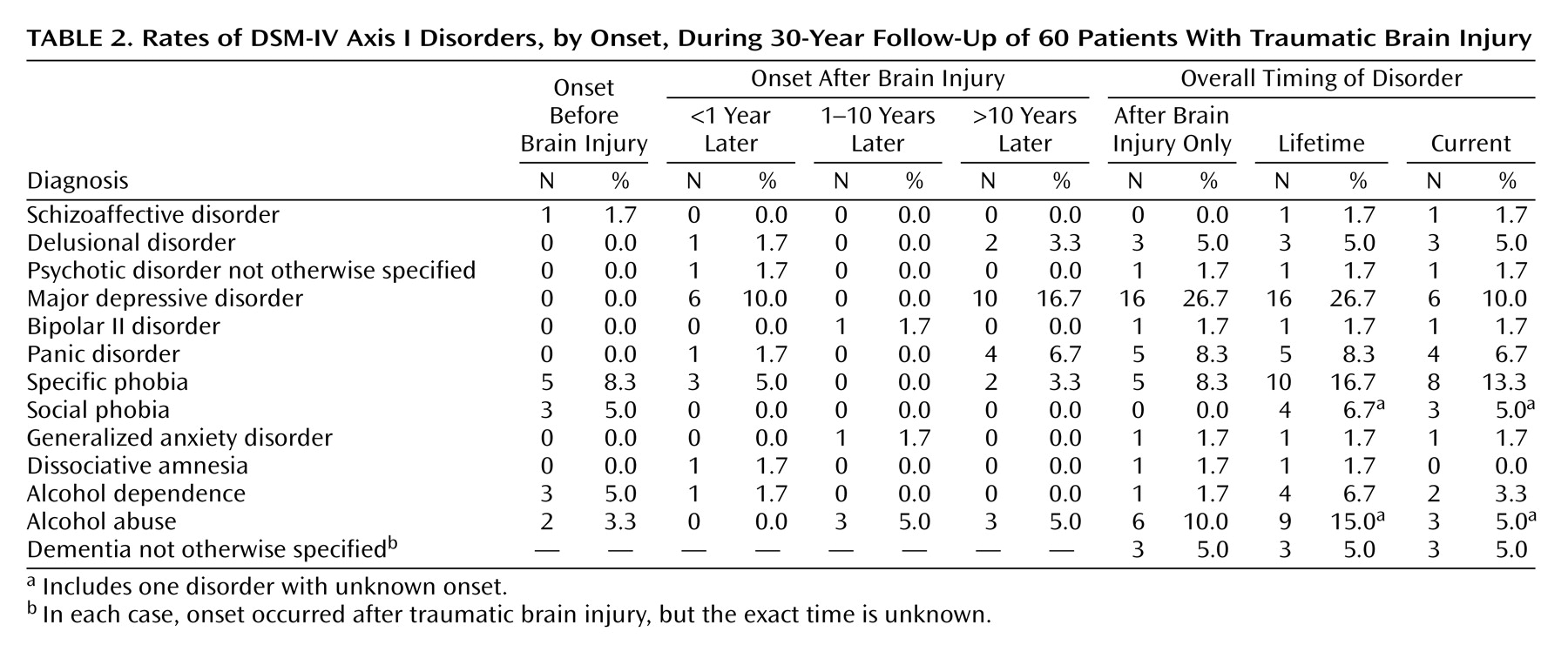
Psychiatric interviews were conducted by a research psychiatrist (S.K.), who was trained to use the instruments. If the interview was considered unreliable (in five patients, 8.3%), the information was checked with a relative. Current (previous month) and lifetime DSM-IV diagnoses of axis I disorders were made on a clinical basis with the aid of the Schedules for Clinical Assessment in Neuropsychiatry interview (version 2.1)
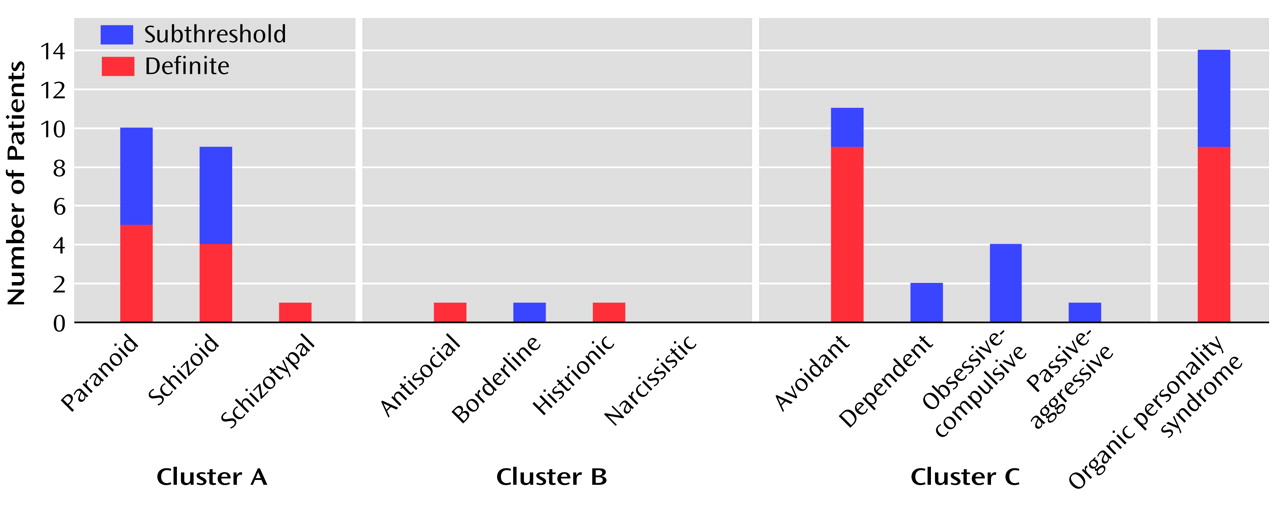
(19). Personality disorders were assessed independently of axis I disorders with the Structured Clinical Interview for DSM-III-R Personality Disorders (SCID-II)
(20). We used the DSM-III-R version of SCID-II, because the DSM-IV version of the screening questionnaire was not available in Finnish at the time of our study. A personality disorder was rated as subthreshold if a patient met all but one of the required criteria. Organic personality syndrome was assessed according to DSM-III-R criteria, and it was divided into the following subtypes: labile, aggressive, disinhibited, apathetic, paranoid, and combined. It was rated as definite if it caused clinically significant distress or disability; otherwise it was rated as subclinical. In three of 14 patients (21.4%) given diagnoses of definite or subclinical organic personality syndrome, a relative was reached to confirm the personality change. For the remaining 11 patients, an informant was not available (N=7) or the patient forbade the attempt to contact an informant (N=4). The diagnosis of organic personality syndrome was not applied to patients with dementia.
Cognitive functioning was evaluated by a research psychologist (L.H.) with the Mild Deterioration Battery
(18), which measures verbal, visuomotor, and episodic memory performance. The Mild Deterioration Battery consists of eight tests: similarities, digit span, digit symbol, and block design from the Wechsler Adult Intelligence Scale
(21), the Benton visual retention test
(22), immediate recall of 30 paired word associates, and naming time and immediate recall for 20 common objects. A patient received 1 deterioration point if his or her performance on any of the eight tests was 1.5 standard deviations below the norm
(18), 2 points if the score was 2.0 standard deviations below the norm, and 3 points if the score was 3.0 standard deviations below the norm. Thus, the maximum total score on the Mild Deterioration Battery was 24 points. On the basis of the total score, each patient was classified as normal (0–1 points), mildly impaired (2–4 points), moderately impaired (5–8 points), severely impaired (9–13 points), or very severely impaired (14 points or more); very severely impaired usually corresponds to clinical dementia. Cognitive impairment was also screened with the Mini-Mental State Examination (MMSE)
(23). The Mild Deterioration Battery and MMSE were not completed for two patients.
Descriptive statistics, such as means, standard deviations, ranges for continuous variables, and frequencies and percentages for categorical variables were used to assess the patients’ background characteristics. To test for differences in categorical variables, chi-square tests or, if necessary, Fisher’s exact tests were applied. Continuous variables were analyzed with one-way ANOVA. To avoid multiplicity, Bonferroni adjustment was used. We calculated 95% confidence intervals (CIs) based on binomial distribution for the rates of psychiatric disorders. Statistical analyses were conducted with SAS statistical software
(24). A two-sided p value of less than 0.05 was considered statistically significant.
Discussion
The main finding of the present study was the high rate of most axis I and II disorders during the 30 years after traumatic brain injury. At present, we do not have epidemiologic data on the prevalence of psychiatric disorders in Finland according to DSM-III, DSM-III-R, DSM-IV, or ICD-10 criteria. However, in our study group, rates of both lifetime and current axis I disorders were significantly higher than the respective prevalences in the population-based Epidemiologic Catchment Area (ECA) survey
(25), which used DSM-III criteria; the rates of lifetime disorders in our study and the ECA survey were 61.7% (95% CI=48.2%–73.9%) and 32.7%, respectively, and the rates of current disorders were 40.0% (95% CI=27.6%–53.5%) and 15.7%. These findings suggest that traumatic brain injury not only temporarily disturbs brain function but may cause decades-long or even permanent vulnerability to psychiatric disorders in some individuals. Correspondingly, Achte et al.
(2) found a latency period of more than 10 years in 42% of the cases of psychosis after traumatic brain injury.
In the present study group, the rates of both lifetime (26.7%, 95% CI=16.1%–39.7%) and current (10.0%, 95% CI=3.8%–20.5%) major depression were significantly higher than the prevalences in the ECA survey (5.9% and 2.3%, respectively)
(25). Our findings are in line with earlier reports of high rates of major depression after traumatic brain injury
(1,
3–
5,
8–10). Major depression was observed not only at the early stage after traumatic brain injury but throughout the 30-year follow-up. In accordance with findings in previous studies
(1,
4,
5,
9), its occurrence was not significantly related to the severity of the brain injury. There were no patients with dysthymia among our subjects, whereas the rate of dysthymia after traumatic brain injury reported in earlier studies has ranged from 2% to 14%
(1,
4,
5,
9). It is possible that brain damage may increase vulnerability to major depression more than susceptibility to dysthymia. The rate of bipolar II disorder (1.7%) was somewhat low compared with the rates of 2% to 17% reported earlier
(3,
7–9).
The lifetime and current rates of panic disorder, 8.3% (95% CI=2.8%–18.4%) and 6.7% (95% CI=1.9%–16.2%), respectively, were significantly higher than the prevalences in the ECA survey (1.6% and 0.5%)
(25). They support previous reports of an increased rate after traumatic brain injury
(1,
8–10). Unlike panic disorder, generalized anxiety disorder was unexpectedly rare in our subjects, compared to earlier findings
(1,
6,
8–10). We did not find patients with PTSD, a finding contrary to recent reports on its occurrence after traumatic brain injury
(9,
10,
12). It is possible that in our patients the heterogeneous symptoms of PTSD did not cluster together 30 years after the accident or that the patients unconsciously avoided recalling symptoms associated with remote traumatic memories.
Lifetime alcohol abuse or dependence was found in 21.7% of the subjects (95% CI=12.1%–34.2%), and current alcohol disorders were present in 8.3% (95% CI=2.8%–18.4%). These figures do not differ significantly from the prevalences of the ECA survey (13.5% and 2.8%, respectively)
(25). Our results are in line with two previous reports
(1,
10), but higher rates have been reported after traumatic brain injury for alcohol abuse in one small-scale study
(8) and for substance use in another study
(9). The rarity of drug use in Finland before the 1990s explains its absence in our study group. Moreover, the mean age of our patients was high, 61 years, and the prevalence of alcohol abuse declines with advancing age because of increased mortality and remissions
(26).
The rates of lifetime and current psychotic disorders were both 8.3% (95% CI=2.8%–18.4%). They were significantly higher than the prevalences in the ECA survey (1.5% and 0.7%, respectively)
(25). Five percent of our patients (95% CI=1.0%–13.9%) had delusional disorder, whereas the estimate for its population prevalence is only 0.03% in DSM-IV. Our figures are in accordance with the rate of 9% for psychoses (2% for paranoid psychoses) after traumatic brain injury found in an early study by Achte et al.
(2). In more recent studies
(3,
10), only a rate of 1% for schizophrenia after traumatic brain injury was reported. In our subjects, paranoid features were also common on axis II, as was reflected in the rate of 16.6% for definite or subthreshold paranoid personality disorder. Two of the three patients with delusional disorder also had dementia, and both patients had delusions of persecution. About one-half of patients with Alzheimer’s disease have delusions, usually persecutory
(27). It is probable that after traumatic brain injury, decreased prefrontal capability to process information makes patients prone to paranoid interpretations.
All eight patients with very severe cognitive impairment were male. This finding cannot be explained by alcohol use in men, because the presence of very severe cognitive impairment according to the Mild Deterioration Battery was not associated with lifetime alcohol abuse or dependence (Fisher’s exact test, p=1.00). Nor were there significant differences in the severity of brain injury between men and women (Fisher’s exact test, p=0.24). The neuroprotective influences of estrogen and progesterone
(28) can, at least partially, explain this more favorable outcome after traumatic brain injury in women.
In population studies
(29–
31) the total prevalence of personality disorders has ranged from 5.9% to 13.5%. The difference in the rate of personality disorders between our study (23.3%; 95% CI=13.4%–36.0%) and the majority of these population studies was substantial. This high rate in our elderly patients is even more striking considering that the prevalence of personality disorders declines with age
(30,
32). Our figure falls below the rates for personality disorders after traumatic brain injury reported by Van Reekum et al.
(8) (38%) and Hibbard et al.
(11) (66%). However, our summed rate of SCID-II personality disorders and organic personality syndrome (30.0%) is close to the former figure. In our study, the most common personality disorders were avoidant (15.0%, 95% CI=7.1%–26.6%), paranoid (8.3%, 95% CI=2.8%–18.4%), and schizoid (6.7%, 95% CI=1.9%–16.2%) personality disorder, while in the population studies, the upper limits of the ranges of prevalences for these disorders have been 1.6%, 7.3%, and 1.6%, respectively
(29–
31). Also, Hibbard et al.
(11) reported that avoidant (26%) and paranoid (26%) personality disorders are common after traumatic brain injury. It seems that traumatic brain injury can expose some individuals to social anxiety, suspiciousness, and detachment. There were only two patients (3.3%) with definite cluster B personality disorders in our group. This is in contrast to the findings of Hibbard et al.
(11), who found borderline personality disorder to be the most prevalent disorder after traumatic brain injury (34%). However, in our study, the category of organic personality syndrome that we applied resulted in a group that contained several individuals with labile and disinhibited features resembling behavior in borderline personality disorder. The high age of our patients may also have influenced the number of cluster B disorders, as they tend to decline with advancing age
(33).
Organic personality syndrome was relatively common (15.0%, 95% CI=7.1%–26.6%) but clearly less prevalent than personality change in earlier studies (49%–80%)
(14–
16). However, these figures have probably included patients with all kinds of axis II disorders. The rate of 32% for ICD-10 organic personality disorder reported recently by Franulic et al.
(17) is closer to our findings. The severity of brain injury was not associated with the presence of organic personality syndrome, which is in line with the findings of Franulic et al.
(17). Although about half of our patients with organic personality syndrome also had an SCID-II personality disorder, we regard organic personality syndrome as a relevant and useful diagnostic category.
The strengths of our study include the assessment of both axis I and II psychiatric disorders with structured instruments, evaluation of cognitive functioning by a sensitive neuropsychological test battery, and to our knowledge, the longest follow-up ever reported. This study has also some limitations. First, although the subjects drawn from the original group were representative, the original patients were referred for neuropsychological evaluation on a clinical basis. For that reason, our conclusions may not be generalizable to all patients with traumatic brain injury. Second, because of incomplete medical records and the lack of systematic neuroradiological examinations at the time of the injury, the information on the nature and location of brain injury and consequently on their association with the development of psychiatric disorders remained insufficient. Third, the reliability of retrospective diagnoses may have been compromised by patients’ memory disturbances. Fourth, as our patients were aged, there were seldom informants available who could report changes following traumatic brain injury about 30 years ago. Therefore, interviewing informants systematically was not possible, which is a limitation particularly in the diagnosis of personality change. Fifth, the small number of subjects and the lack of an age-matched comparison group are shortcomings.
In summary, our results suggest that traumatic brain injury can cause decades-long or even permanent vulnerability to psychiatric disorders in some individuals. Personality disturbances, which were common among our patients, can be difficult to detect and may impair compliance with rehabilitation. Therefore, psychiatric evaluation and follow-up should be included in the routine treatment of traumatic brain injury.