Neurobiological theories of attention deficit hyperactivity disorder (ADHD) have predominantly focused on the prefrontal cortex and the basal ganglia. This is logical given the association of frontal lobe hypofunction with impulsive behavior, working memory, and deficits of executive function
(1). The basal ganglia are well known to be involved in the regulation of locomotor activity, and both regions are primary targets of the ascending dopamine system
(2), which is directly affected by stimulant medications
(3). However, the largest morphometric studies of ADHD children have consistently found that both boys and girls with ADHD have smaller posterior-inferior lobules of the cerebellar vermis
(4–
7) than healthy children. This is particularly interesting as the cerebellum receives dopamine projections from the ventral tegmental area
(8,
9) and the posterior-inferior lobules of the cerebellar vermis have the highest concentration of dopamine transporters in the primate vermis
(10). Furthermore, there are reciprocal loops that interconnect a large and diverse set of cerebral cortical areas, including the prefrontal cortex, with the basal ganglia and cerebellum by way of the pons, dentate nucleus, and thalamus
(11,
12). The vermis, through its fastigial projections to the ventral tegmental area and locus ceruleus, exerts strong effects on the turnover of dopamine and noradrenaline in the caudate and nucleus accumbens
(13–
19). Stimulation of the hook bundle, a projection pathway in the white matter of the cerebellum, containing fibers from the caudal fastigial nucleus, which receives direct Purkinje cell efferents from the posterior-inferior vermis
(20,
21), elicits locomotor activity in the decerebrate cat
(22). In short, there are compelling reasons to hypothesize that the cerebellar vermis may be involved in both the pathophysiology of ADHD and therapeutic response to stimulant medications.
The purpose of the present study was to investigate the effects of methylphenidate on the cerebellar vermis of children with ADHD by using T
2 relaxometry, a novel functional magnetic resonance imaging (fMRI) procedure developed to assess changes in steady-state blood flow with long-term drug administration
(23). Previously, we observed that daily treatment with high-dose methylphenidate changed T
2 relaxation time in the putamen, supporting the link between dysfunction in cortical-striatal-thalamic circuits and the capacity to inhibit motor activity and sustain attention
(23).
Although stimulants are known to exert robust effects on catecholamine systems, imaging studies have often failed to reveal reproducible medication effects. For example, Matochik et al.
(24) found no consistent effect of the long-term administration of methylphenidate or dextroamphetamine on the regional brain metabolism of adult ADHD patients using PET. Similarly, Ernst et al.
(25) found no consistent effect of intravenous dextroamphetamine on the global or regional metabolic rates of adult ADHD subjects, even though the drug produced robust effects on attention. Global metabolic rates changed substantially in most patients after treatment but in both directions. Volkow et al.
(26) found that methylphenidate increased frontotemporal metabolism in subjects with a high availability of dopamine D
2 receptors and decreased metabolism in subjects with a low availability of D
2 receptors. These findings suggest that stimulants exert bidirectional effects on blood flow or metabolism, which may depend on individual differences in dopamine receptor density or other factors. We found that the magnitude and direction of methylphenidate effects on T
2 relaxation time in the putamen were highly dependent on the subject’s hyperactivity (capacity to sit still when not taking medication)
(23), which may be related to individual differences in dopamine receptor density or function as has been observed for mice
(27) or dopamine transporter density or function as observed in primates
(28). Hence, we coupled fMRI investigation with objective laboratory assessment of the child’s capacity to sit still and pay attention while receiving placebo and during treatment with low, intermediate, and high doses of methylphenidate.
Method
Subjects
Ten boys with ADHD (mean age=9.3 years, SD=1.6) and six healthy comparison boys (mean age=10.2 years, SD=1.5) were studied. McLean Hospital’s institutional review board approved this protocol, and written informed consent was obtained from parents or guardians. In addition, the fMRI procedures were carefully explained to the children, who also assented to participate. Children with ADHD were screened by use of a structured diagnostic interview (the Schedule for Affective Disorders and Schizophrenia for School-Age Children—Epidemiologic Version
[29]) and were included if they met criteria for ADHD and had at least six of nine symptoms of inattention or hyperactivity-impulsivity. Children then took part in a triple-blind (parent, child, and rater), randomized, placebo-controlled study of the effects of methylphenidate (0.0, 0.5, 0.8, or 1.5 mg/kg per day in two divided doses) on activity and fMRI. Children were treated continuously for 1 week with placebo or with a specific dose of methylphenidate. They were tested on day 7 for drug response by using objective measures of activity immediately after fMRI. On test days, parents were instructed to give the afternoon dose 1 hour before the fMRI appointment. Medication administration was assessed at each visit to ensure that time between ingestion and testing was held constant for each subject throughout the study. Within each subject there was an average of 28 minutes of variation in fMRI start times between the different dose conditions. Objective assessment of attention and activity followed fMRI by an average of 42 minutes (SD=39).
fMRI Procedures
Images were acquired by using a 1.5-T magnetic resonance scanner (Signa, General Electric Medical Systems, Milwaukee) equipped with a whole-body resonant gradient set capable of echo-planar imaging (Advanced NMR Systems, Inc., Wilmington, Mass.) and a standard quadrature head coil for image detection. During each examination, three categories of images were obtained: 1) scout images (typically T1-weighted sagittal images), 2) high-resolution T1-matched axial images through the 10 planes for which maps of T2 were generated, and 3) 32 spin-echo, echoplanar image sets, with TE incremented by 4 msec in each consecutive image set (e.g., TE 1=32 msec, TE 2=36 msec,… TE 32=156 msec) through the same 10 axial planes (TR=10 msec, slice thickness=7 mm with a 3-mm skip, in-plane resolution=3.125 mm × 3.125 mm, field of view=200 mm).
The 32 TE-stepped images were then transferred to an offline workstation and corrected for in-plane motion by using a modification of the DART image registration algorithm
(30). The DART algorithm provided vertical, horizontal, and rotational values for subject movements during each functional scan. These values, which were within the range for which the registration algorithm has been validated, were significantly different between ADHD subjects and comparison subjects for horizontal and vertical movement on analysis of variance (ANOVA) (x axis: F=8.33, df=1, 14, p=0.01; y axis: F=9.07, df=1, 14, p=0.01). Of interest, there were no differences for ADHD subject movements as a function of methylphenidate dose (F=1.97, df=3, 21, p=0.15). For example, average head movement in the maximal vertical direction for boys with ADHD taking placebo was 1.16 mm, 1.66 mm after high-dose methylphenidate, and 0.12 mm for comparison subjects.
Values of T
2 (x, y) and S (TE=0, x, y) were then calculated on a pixel-wise basis, assuming exponential decay, i.e., ln(S[x, y, n])=ln(S[TE=0], x, y)–TE (n)/T
2(x, y), where (x, y) describes the location of the pixel, n characterizes the echo number, from 1 to 32, and S is the image signal intensity. Linear least-squares regression was used to calculate a single T
2 relaxation time measure for each pixel (x, y). Calculations of regional T
2 relaxation time were made for regions of interest drawn manually under the guidance of conventional T
1-weighted matched MRI images of two to four slices through the cerebellum by using anatomic boundaries observed in T
1-weighted, preliminary T
2 relaxometry maps and an atlas of the cerebellum
(31). Region-of-interest slice selections were based on an assessment of the presence of CSF in order to minimize partial volume artifacts, which result in abnormally elevated T
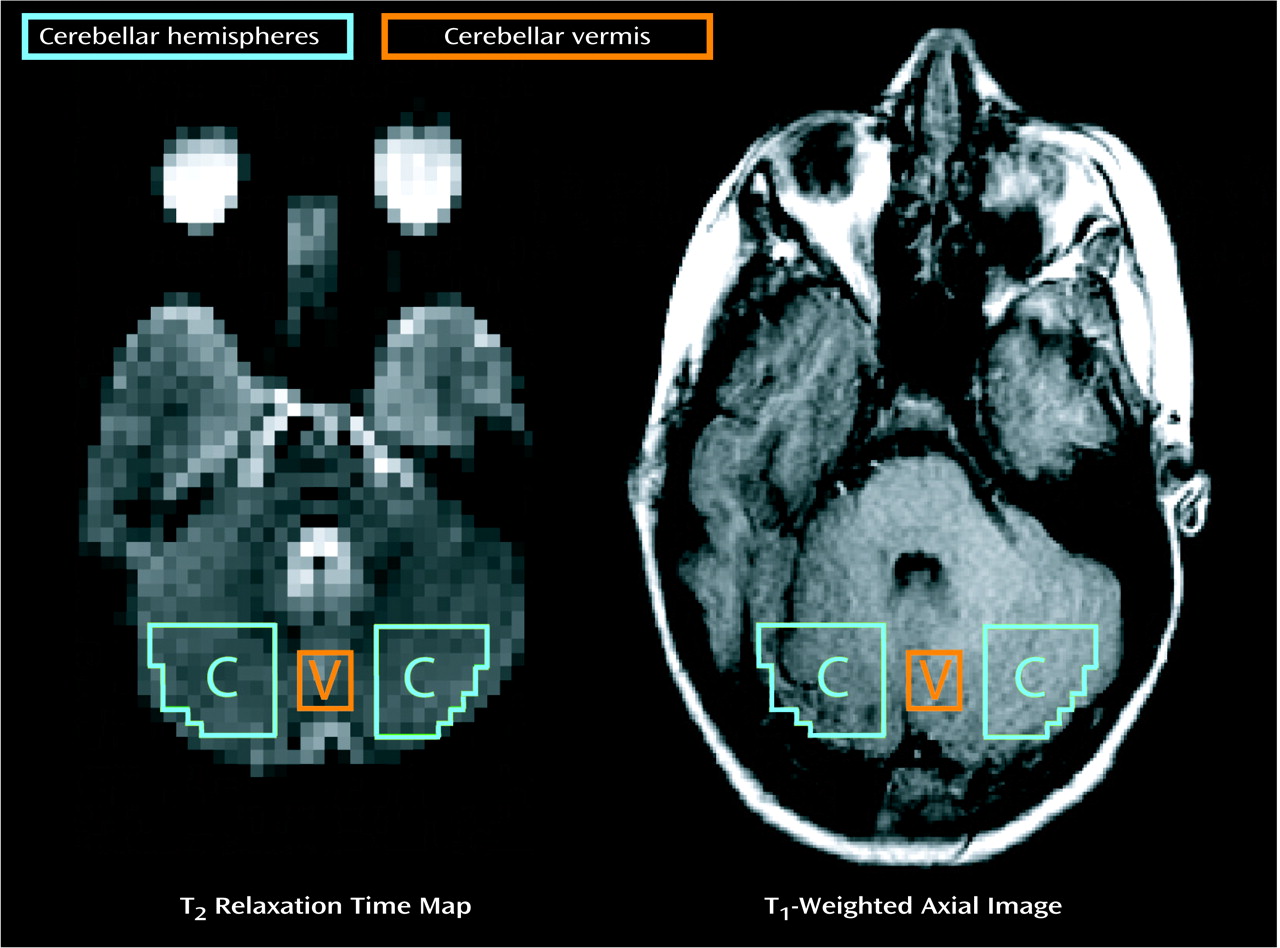
2 relaxation times (values >100 msec were rejected). Sixteen voxel regions of interest in the vermis were conservatively limited to square regions (x=6 mm by y=6 mm) to avoid encroaching into ventricular space and to provide consistency between slices and subjects. Representative cerebellar regions of interest are depicted in
Figure 1. Delineation of regions and analysis of imaging data were performed on coded images by an analyst who was familiar with cerebellar anatomy and was blind to the identity, diagnosis, and treatment conditions of the subject. The coefficient for intrarater reliability, alpha, was 0.987, based on four separate and independent T
2 relaxation time measures from the vermis of seven subjects. Regional T
2 relaxation times were calculated from the median value of all the designated pixels, as the median provides a regional estimate that is less susceptible to contamination by spurious values from CSF than does the mean. Regional T
2 relaxation time values were averaged for all slices within subjects, and differences between placebo and drug conditions were then calculated.
Assessment of Activity
To accurately gauge the effects of methylphenidate on cerebellar blood flow, it was important before scanning to precisely assess individual differences in the ability to sit still and pay attention. A newly developed procedure was used in which subjects were challenged to attend to a monotonous but demanding computerized continuous performance task, during which head movements were recorded by using infrared motion analysis
(23,
32). The optical tracking system recorded fidget-induced vertical and horizontal displacements of a reflective head marker to a resolution of 0.04 mm. Measures of immobility were derived from marker movement by calculating the average time between movements during the 15-minute test. A movement was defined to occur whenever the marker moved >1 mm from the position of the previous movement.
Results
Conventional analysis of cerebellar or vermal T2 relaxation time failed to reveal any significant effect of dose with repeated measures ANOVA (F=1.17, df=3, 27, p=0.34). Some subjects had an increase in T2 relaxation time, and others had a decrease. However, the magnitude and direction of change was not random but varied directly with each subject’s degree of hyperactivity when unmedicated. Analysis of covariance with motor activity as a covariant revealed a robust effect of methylphenidate treatment on vermal T2 relaxation time (F=4.85, df=3, 24, p=0.009), along with a significant dose-by-activity interaction (F=3.90, df=3, 24, p=0.02). Further, the effect of dose was linear (F=15.14, df=1, 8, p=0.005). No effect of methylphenidate treatment was observed on T2 relaxation time in the adjacent cerebellar hemispheres (dose: F=0.88, df=3, 21, p>0.40; dose by activity: F=0.47, df=3, 21, p>0.70).
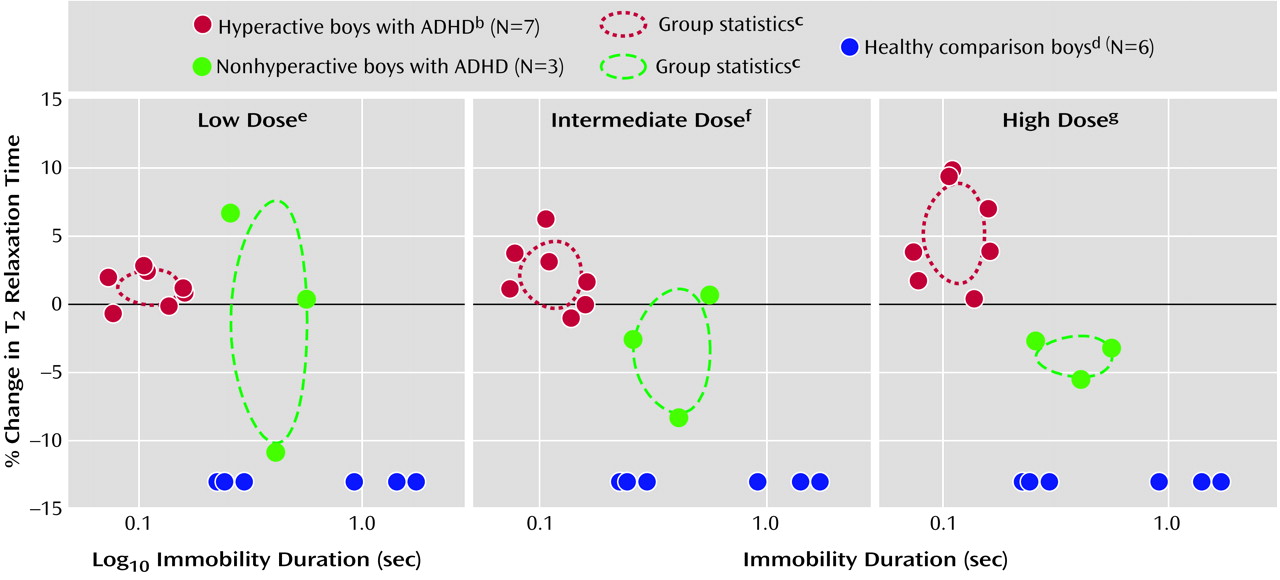
Boys who spent the least time sitting still (e.g., who were the most hyperactive) demonstrated a positive dose-dependent change in T
2 relaxation time (red circles in
Figure 2). A negative dose-dependent change in T
2 relaxation time was observed in boys who sat still longer (green circles in
Figure 2). Vermal T
2 relaxation time increased 1.2%, 2.1%, and 5.1% at low, intermediate, and high doses of methylphenidate, respectively, in seven boys who were at least 20% more active than the most active comparison subject (blue circles in
Figure 2). In contrast, T
2 relaxation time decreased an average of 1.3%, 3.5%, and 3.9% in the three least-active boys with ADHD. Percent changes in T
2 relaxation time for hyperactive and nonhyperactive boys were significantly different by repeated measures ANOVA for intermediate and high doses but not low doses of methylphenidate (
Figure 2). There was no significant association between percent change in T
2 relaxation time and percent change in activity with methylphenidate. However, at the highest dose tested, there was a modest correlation (r=0.45, df=8, p=0.19), which would have been significant if an apparent outlying point had been eliminated (r=0.72, df=7, p=0.02).
There was a significant association between the objective measures of time spent immobile taking placebo and number of DSM-IV symptoms of hyperactivity reported during the clinical interview (r=0.72, df=10, p=0.01). However, clinical rating of activity was a weak covariate that failed to reveal a significant effect of methylphenidate on T2 relaxation time (F=0.66, df=3, 23, p>0.50).
Discussion
The highest dose of methylphenidate produced on average a 5.1% increase in T
2 relaxation time in the children with ADHD who were objectively hyperactive and a 3.9% reduction in T
2 relaxation time in the three ADHD children who were not objectively hyperactive. Are these percent changes biologically important? First, while these appear to be small percentage differences, they are quite strong and correspond to an effect size (Cohen’s d) of 2.13 (95% confidence interval=0.82–3.44) in the hyperactive subjects. A linear regression relationship between vermal T
2 relaxation time and relative cerebral blood volume has been established by using dynamic susceptibility contrast MRI
(33) in nine healthy 18–22-year-old adults
(34) under resting conditions. Vermal relative cerebral blood volume values ranged from 0.7 to 2.0 (arbitrary units), while T
2 relaxation times ranged from 90.0 to 95.5 msec. Hence, a 4-msec change in T
2 relaxation time may be associated with a very marked change in relative cerebral blood volume. Precise estimation of relative cerebral blood volume changes should not be inferred, as this correspondence was observed in unmedicated adults and not medicated children, but it does suggest that this degree of change in T
2 relaxation time should be associated with a biologically important change in relative cerebral blood volume.
These findings indicate that methylphenidate exerts a robust dose-dependent effect on the paramagnetic properties of the cerebellar vermis, which presumably is the result of alterations in regional blood flow and volume. However, both the magnitude and direction of the effects were entirely dependent on the subject’s basal level of hyperactivity or capacity to sit still. Failure to incorporate this intervening variable in the analysis completely obscured the effect. This may help to explain why previous efforts have failed to find consistent effects of methylphenidate or dextroamphetamine on regional brain metabolism
(24,
25). Conceivably, precise quantitative measures of basal (unmedicated) activity or rate would have provided a key to understanding why regional brain metabolism increased in some subjects and decreased in others.
Preclinical research has frequently suggested that stimulants work in a rate-dependent manner, exerting behavioral effects that are inversely correlated to the basal rate of the behavior
(35). That is, stimulants tend to decrease behaviors that normally occur at high rates and to increase behaviors that occur at low rates. Rate dependency contrasts with clinical reports that suggest that stimulants exert the same qualitative effect on all individuals
(36,
37). However, those reports also acknowledge prominent quantitative differences in degree of response. The hypothesis that stimulants exert rate-dependent effects is supportive of observations by Vaidya et al.
(38), found when using blood-oxygen-level-dependent fMRI, that stimulants exert qualitatively different effects on activation of the striatal system of normal comparison subjects and children with ADHD. Our primary finding—that methylphenidate effects on T
2 relaxation time in the vermis depend strongly on basal activity levels—is wholly consistent with a rate-dependency hypothesis and suggest a possible neurobiological substrate for these behavioral effects.
Although methylphenidate acts primarily as a dopamine transporter antagonist, it also has affinity for the noradrenaline transporter
(39,
40), and it is possible that noradrenaline neurons play a role in the response observed. Further binding studies in human tissue are essential to explore these issues.
The effects of methylphenidate on T
2 relaxation time in the cerebellar vermis are consistent with a large number of studies implicating the cerebellum as a modulator of forebrain dopamine outflow
(18,
19,
41,
42). Dopamine receptors
(43,
44) and transporter immunoreactivity
(10) have recently been observed in the primate cerebellum, and these observations are consistent with direct effects of stimulants on the cerebellum. Although early studies implicated the cerebellum as a modulator of dopaminergic outflow, only a few imaging studies have provided information on the interaction of stimulant drugs with the cerebellum
(26,
45–48). In two studies, Volkow et al.
(26,
46) reported consistently increased relative metabolic activity in the cerebellum after administration of intravenous methylphenidate (0.5 mg/kg) in normal adults. In the first study, the change in methylphenidate-stimulated activity from placebo activity correlated strongly with cerebellar dopamine receptor availability
(26).
Other preclinical and clinical studies provide additional support for an association between the cerebellar vermis, hyperactivity, and stimulant action. For example, developmental lesions or mutations that have been associated with the transient development of hyperactivity in rats
(49) and mice
(50) also result in pathology of the midline cerebellum. When the cerebellum of neonatal rats is sequentially exposed to X-rays during production of granule cells targeted for the posterior-inferior vermis, these animals express greater spontaneous wheel running as young adults than do nonexposed rats
(49). Mice homozygous for the “pallid” recessive mutation (phenotypically lack vestibular otoliths and display behavioral hyperactivity) are calmed by administration of amphetamine
(50). In a clinical positron emission tomography imaging study of adults with ADHD who were retrospectively diagnosed with childhood hyperactivity, Schweitzer et al. (personal communication) observed blood flow changes in lobule VIII that were responsive to methylphenidate administration. In summary, clinical and preclinical behavioral, correlational, and imaging studies support an association among the cerebellar vermis, hyperactivity, and stimulant action.
Although the findings reported here are preliminary in nature and require further replication, taken together, they implicate the cerebellar vermis as an important brain region that may be directly involved in the pathophysiology of ADHD and in rate-dependent stimulant effects on behavior. Further research will be needed to clarify the relationship between vermal size, vermal blood flow, stimulant response, and the developmental pathophysiology of ADHD.