1. Patients with schizophrenia will demonstrate poor item-specific recollection and will rely on familiarity to make old/new decisions, leading to a greater degree of false recognition of repeated foils than seen in healthy adults.
2. Patients with schizophrenia will not utilize the distinctiveness heuristic and will therefore not demonstrate the normal pattern of false recognition suppression after picture encoding, as compared to word encoding.
Method
Subjects
Forty male patients with DSM-IV-defined schizophrenia were recruited from our outpatient clinic in Boston. Confirmation of diagnosis and determination of schizophrenic subtype was made for all patients by clinic psychiatrists, who administered the psychotic disorders module of the Structured Clinical Interview for DSM-IV Axis I Disorders, Patient Edition (SCID-P)
(24). All patients were taking a stable dose of antipsychotic medication (12 were taking conventional neuroleptics, 27 were taking atypical neuroleptics, and one was taking lithium monotherapy) and were not withdrawn from their medication for the purposes of the study. The mean dose of medication was equivalent to 557 mg of chlorpromazine. In addition, six of the patients were taking an anticholinergic medication (four were taking benztropine, and two were taking trihexyphenidyl) at the time of study participation. Patients were excluded if they had a history of significant neurological illness (seizure disorder, head trauma, stroke) or if they met criteria for alcohol or other substance abuse within the past 3 months.
Thirty-two age-matched male subjects, recruited by means of advertisement from the local community, served as a comparison group. Comparison subjects were free of any axis I psychiatric condition as determined by the SCID-P (administered by A.P.W.) and did not have a history of major medical or neurological illness or of substance abuse. None of the comparison subjects was taking psychotropic medication. One of the 32 comparison subjects had a first-degree relative (brother) with a history of schizophrenia.
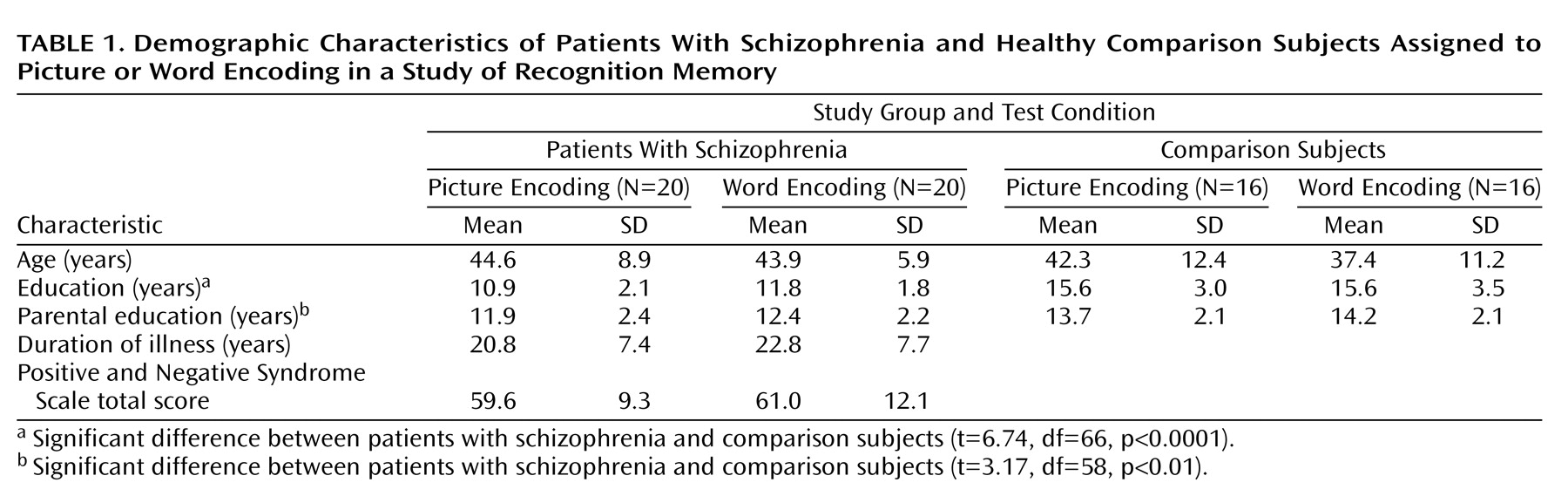
Comparison subjects had a higher level of formal education and a greater mean parental level of education than the schizophrenia group (
Table 1). There were no significant within-group differences in age, education, parental education, duration of illness, or symptom severity (as defined by the Positive and Negative Syndrome Scale
[25]) between subjects assigned to the picture- or word-encoding conditions.
Before enrollment of subjects, the protocol was approved by the institutional review boards of both the Massachusetts General Hospital and the state Department of Mental Health. After a complete description of the study, informed, written consent was obtained from all participants.
Materials and Study Design
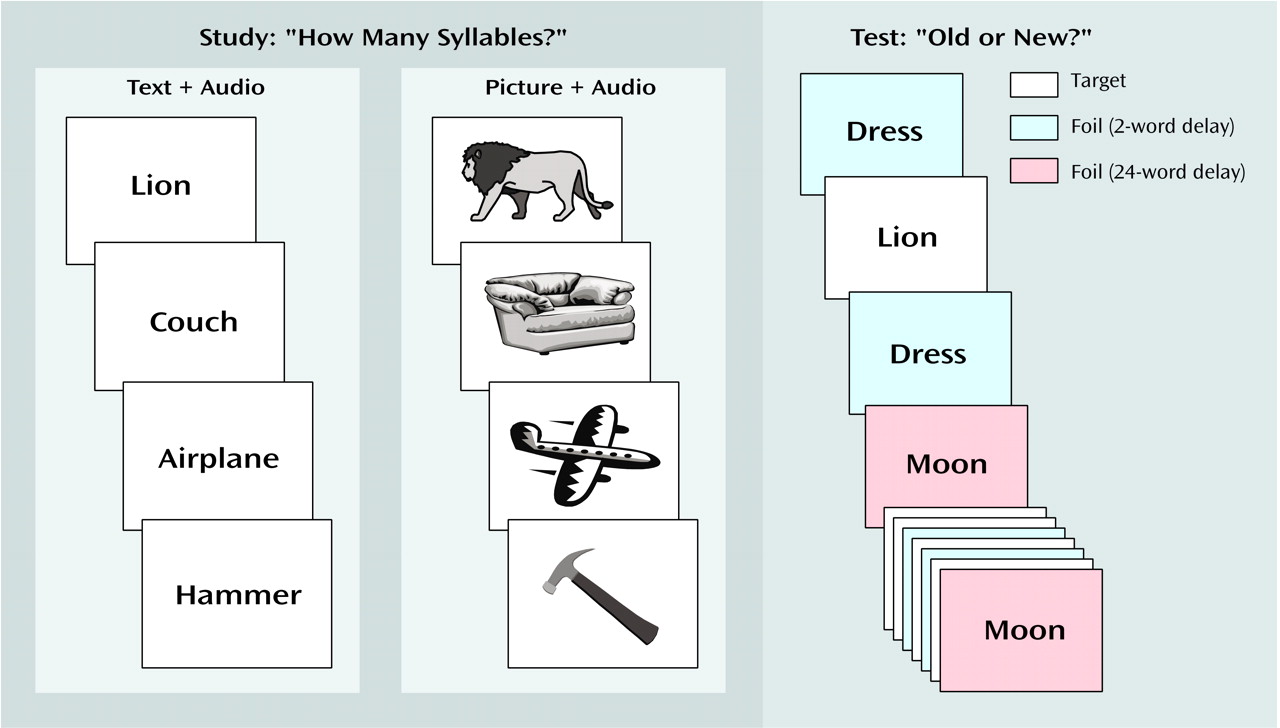
The experimental paradigm was an old/new recognition memory test
(11–
14,
26). The stimuli consisted of 120 line drawings or their corresponding verbal labels
(27). Sixty items were studied, either as pictures or words, and were then seen as old items at the time of the test. The other 60 items served as new foils at the time of the test, with half of these items repeating at either a two-word or a 24-word delay (
Figure 1).
Participants were alternatively assigned to either the picture- or word-encoding condition. Stimuli were presented on a computer screen, concurrent with auditory presentation of the item name by means of headphones. Subjects were told to indicate the number of syllables (one or two) in the study item by using a self-paced button press and were unaware of the subsequent memory test.
In the test phase, which occurred immediately after the items were studied, all items appeared in word format without auditory presentation, regardless of study condition. Participants were informed that they would now see old words (i.e., items seen during the syllable-counting phase) and new words (i.e., items not seen previously) and would be asked to distinguish between the two by using a self-paced button response. They were also told that the new items would repeat so that they would be seen twice. They were instructed to continue to consider these repeating new words as new, as they were not encountered during the syllable-counting phase of the experiment.
Statistical Analysis
Correct recognition of old items (hit rate) and the false characterization of new foils as old during their initial presentation (false-alarm rate) were analyzed by using a two-way analysis of variance (ANOVA) (group: schizophrenia versus comparison; encoding condition: picture versus word). Old/new discrimination accuracy and response bias were measured with the signal detection parameters of discrimination (d′) and bias (C), respectively
(28), on the basis of the hit rate and false-alarm rate of each subject. As hit rates of 0 or 1 lead to undefined values in these analyses, the data were transformed in standard fashion by adding 0.5 to each individual’s hit total and dividing by N + 1 (rather than N)
(29). Values of d′ and C were then also analyzed by using a two-way ANOVA (group and encoding condition).
To determine the degree of false recognition of the repeated foils, false recognition rates after the two-word and 24-word delay were first corrected by subtracting out the baseline rate of false recognition of new words at initial presentation. This corrected rate of false recognition was then analyzed by using repeated measures ANOVA, with group and encoding condition as between-subject factors and length of delay (two-word versus 24-word) as a within-subject factor.
To examine the potential confound of mean parental education on memory performance, we ran a simple linear regression analysis of mean parental education versus overall discrimination accuracy (d′) and corrected rate of false recognition. We examined the potential confound of encoding performance (syllable counting) on subsequent memory in a similar fashion. We studied the potential impact of medication status on performance in two ways: 1) by means of a simple linear regression analysis of neuroleptic dose (in chlorpromazine equivalents) versus d′ and corrected rate of false recognition, and 2) by using t tests to compare these memory performance statistics between patients taking conventional neuroleptics and those taking atypical neuroleptics and between patients taking anticholinergic medications and those who were not.
Results
Hit Rate, False-Alarm Rate, and Signal Detection Analyses
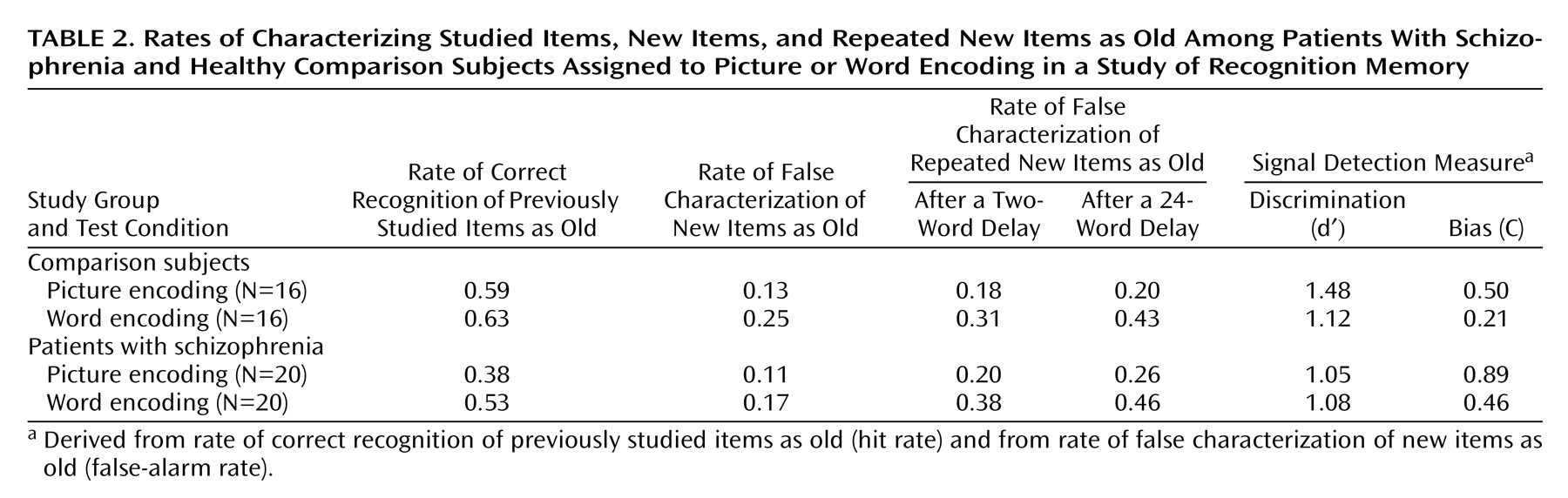
Correct recognition of previously studied items (hit rate) was greater in comparison subjects (mean=0.61, SD=0.19) than patients with schizophrenia (mean=0.45, SD=0.23) (F=9.81, df=1, 68, p<0.005) and tended to be higher after word encoding (mean=0.58, SD=0.20) than after picture encoding (mean=0.47, SD=0.25) (F=3.59, df=1, 68, p=0.06) (
Table 2). There was no interaction of group and encoding condition.
The false recognition of new foils at their initial presentation (false-alarm rate) did not differ significantly between comparison and patient groups (mean=0.19, SD=0.14, and mean=0.14, SD=0.13, respectively). The false-alarm rate was higher after word (mean=0.21, SD=0.13) than picture (mean=0.12, SD=0.13) encoding (F=8.15, df=1, 68, p<0.01), without an interaction of group and encoding condition.
Signal detection analyses indicated that comparison subjects were better at discriminating old from new items, as evidenced by a higher level of d′ (1.30 versus 1.07) (F=4.01, df=1, 68, p=0.05). Encoding condition had no significant effect on old/new discriminability. Schizophrenic subjects had a significantly more conservative response bias (i.e., a greater reluctance to call items old), as evidenced by the higher criterion threshold (C=0.67 versus 0.35) (F=6.37, df=1, 68, p<0.05). Subjects in both groups were more conservative in their responses after picture encoding (C=0.71) than after word encoding (C=0.35) (F=8.04, df=1, 68, p<0.01).
False Recognition of Repeated Foils
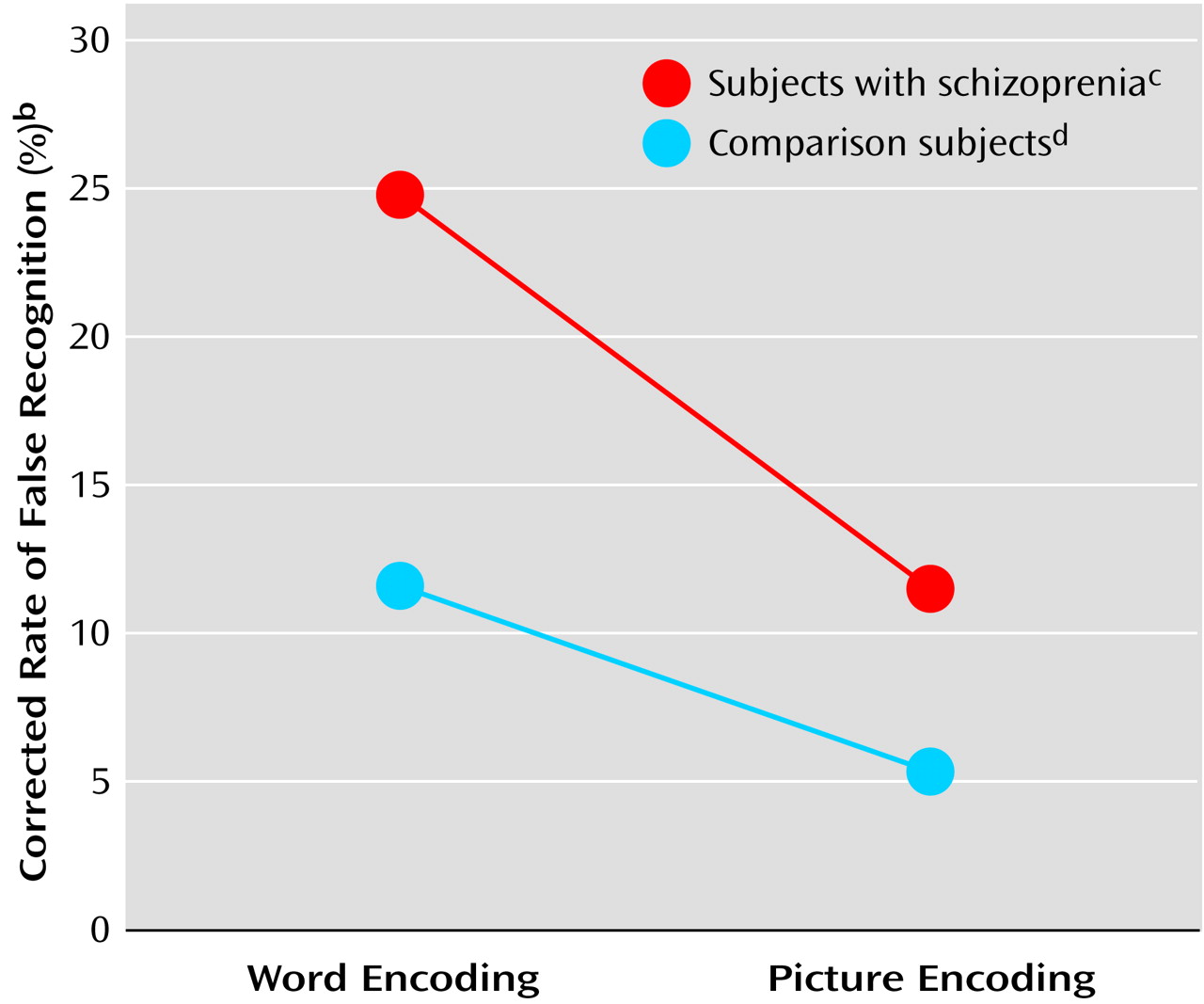
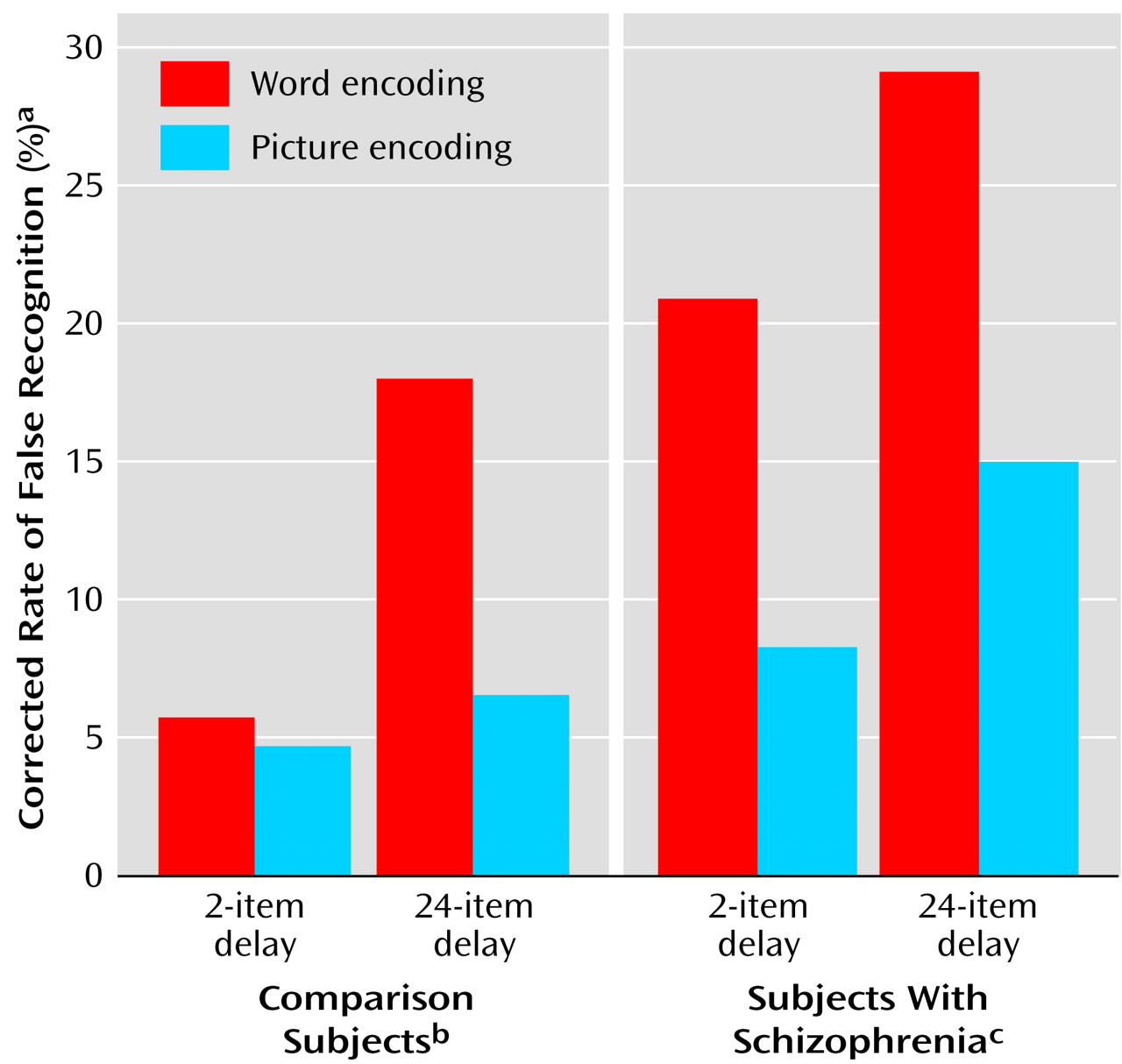
Schizophrenic subjects showed greater false recognition of repeated foils (corrected for baseline rate of false recognition) (mean=0.18, SD=0.16) than the comparison subjects (mean=0.09, SD=0.13) (F=11.28, df=1, 68, p<0.005). The rate of false recognition of repeated foils was lower after picture (mean=0.09, SD=0.1) than word encoding (mean=0.19, SD=0.18) (F=11.72, df=1, 68, p<0.001) in both groups. Despite schizophrenic patients’ higher level of false recognition of repeated foils, both schizophrenic and comparison subjects demonstrated a similar degree of false recognition suppression after picture encoding, compared to word encoding (interaction of encoding condition by group: F=1.45, df=1, 68, p=0.23) (
Figure 2).
In addition to these significant main effects of group and encoding condition, there was also a significant effect of delay, such that longer delay to repetition (i.e., 24 words versus two words) led to a greater degree of false recognition (0.18 versus 0.10) (F=34.43, df=1, 68, p<0.0001). The effect of delay was more pronounced after word than picture encoding (interaction of delay and encoding condition: F=5.81, df=1, 68, p<0.05).
This accentuation of the effect of delay following word-encoding tended to be more pronounced in the comparison group (interaction of delay by encoding condition by group: F=3.26, df=1, 68, p=0.08), owing largely to a heightened degree of false recognition in the schizophrenic cohort at even the shortest delay interval (
Figure 3). Indeed, the degree of false recognition after only a two-word delay was significantly higher in the schizophrenic group than in the comparison group (0.15 versus 0.05) (F=11.32, df=1, 68, p<0.005), particularly after word encoding (for two-word delay, interaction of encoding condition by group: F=4.09, df=1, 68, p<0.05).
We examined the potential confounding influence of mean parental education, encoding performance (syllable counting), and medication status on memory performance. Regression analyses indicated no significant association between mean parental education and either d′ (r=0.07, df=59, p=0.61) or false recognition (r=0.09, df=59, p=0.49). While syllable counting accuracy was slightly higher in the comparison subjects (mean=95.1%, SD=7.3%) than in the patients with schizophrenia (mean=89.3%, SD=12.1%) (t=2.38, df=70, p<0.05), syllable counting accuracy did not correlate with either d′ (r=0.14, df=71, p=0.24) or false recognition (r=0.05, df=71, p=0.71). There was no significant association between medication dose and either d′ (r=0.21, df=33, p=0.25) or false recognition (r=0.09, df=33, p=0.62) within the schizophrenic cohort. Patients taking atypical medication did not differ from those taking conventional medication in either d′ (t=0.03, df=37, p=0.98) or false recognition (t=0.12, df=37, p=0.91). Similarly, patients taking anticholinergic medication did not differ from other patients on either main measure of memory performance (d′: t=1.23, df=38, p=0.23; false recognition: t=0.89, df=38, p=0.38). Finally, given the potential for cognitive impairment even in first-degree relatives of patients with schizophrenia, we reran all analyses after excluding the single comparison subject with a positive family history. This did not affect the significance of any analyses.
Discussion
We found that patients with schizophrenia demonstrated an elevated rate of false recognition of repeated foils, relative to the comparison group. Despite this elevated rate of false recognition, patients with schizophrenia were just as successful in reducing false recognition after picture encoding (compared to word encoding). The former finding indicates a degraded ability to use item-specific recollection to sort old from new events in schizophrenia. The latter finding suggests an intact ability in schizophrenia to improve performance, even in the face of this deficit in conscious recollection.
These results add to a growing body of literature describing impaired verbal memory in patients with schizophrenia. These memory deficits include a decreased ability to identify the temporal and spatial context in which words were learned and an inability to assess the veridical nature of one’s own recollective experience, leading to elevated rates of false recognition
(4,
10). This pattern of verbal memory deficits has been interpreted as evidence for a core cognitive deficit (impaired conscious awareness) and its neural substrate (impaired frontotemporal integration) in schizophrenia
(10,
30–32). Our study provides further support for this type of specific cognitive deficit. We furthermore provide novel evidence that this deficit can be corrected by means of additional information at the time of learning. As verbal memory is a strong predictor of social functioning in schizophrenia
(33), this latter finding may have important clinical implications. Remediation of cognitive impairment, an important approach to the long-term treatment of schizophrenic patients
(34–
36), can rely on intact metacognitive strategies, even in the face of significant cognitive impairment.
Unlike other old/new recognition memory experiments, where reliance on item familiarity can be used to distinguish previously studied targets from novel foils, the paradigm used in this study penalized the use of such a strategy, as both targets and repeated foils are familiar. Correct discrimination of studied items from repeated foils therefore requires the use of a different mnemonic process, namely the recollection of source, or item-specific, information regarding the previous exposure to the stimulus
(11,
12,
15,
37,
38). Ability to recall the temporal context in which the item had been previously experienced (i.e., during the study or test phase) allows for correct distinction between repeating foils and previously studied targets. The significantly greater degree of false recognition displayed by the schizophrenic group suggests an impairment in this recollective process. These results extend previous work demonstrating deficient source monitoring in schizophrenia, including evidence of poor temporal awareness
(39–
42).
The apparent reliance on item familiarity, leading to the mistaken categorization of repeated foils as having been previously studied, also supports prior studies in schizophrenia that have found heightened reliance on familiarity-based memory processes
(9,
10). Interestingly, this pattern of memory performance is similar to that of healthy elderly subjects, who also seem to be prone to basing mnemonic decisions on a feeling of familiarity, leading to a heightened degree of false recognition in this paradigm
(11,
12,
15,
43), as well as in other tasks requiring old/new discrimination
(44–
46). It is not clear which cognitive and neural mechanisms are responsible for the memory impairment in normal aging, but there is growing evidence that older persons utilize different functional brain networks, perhaps to compensate for reductions of efficiency in task-related brain areas
(47,
48).
Patients with schizophrenia demonstrated elevated levels of false recognition to repeated novel stimuli after even a two-word delay, relative to the comparison subjects (
Figure 3). This difficulty in properly categorizing an item in relation to one seen only a few moments earlier may represent a defect in verbal working memory processes in the schizophrenic group, a well-documented deficit in this population
(49–
52). Unlike patients with schizophrenia, comparison subjects may be able to hold the previous representation of the foil “on-line” and therefore may be able to quickly and reliably categorize the repeating item as new.
Despite the elevated rate of false recognition to repeated foils, schizophrenic subjects were able to suppress the rate of false recognition after viewing pictures (as compared to words) in the study phase. As encoding condition (picture versus word) had no significant impact on old/new discriminability (as measured by d′), the finding of lower rates of false recognition of repeated items after picture encoding cannot be simply attributed to the greater ease of processing pictorial as compared to verbal information. This false recognition suppression after picture encoding, seen in prior studies of healthy adults, is ascribed to the use of a metacognitive strategy called the “distinctiveness heuristic”
(15,
18,
19). Contrary to our initial hypothesis, it appears that this type of heuristic was operating in both the comparison and schizophrenic cohorts. This suggests that some mental heuristics may remain intact in schizophrenia, despite impairments in other metacognitive skills.
In line with prior studies of recognition memory in schizophrenia, this study found that patients demonstrated a slight impairment in overall recognition memory, as evidenced by a lower hit rate (correct recognition of previously studied items) and decreased discrimination accuracy (d′)
(4,
9,
10). In contrast to patients in prior reports of response bias in schizophrenia, the patients in this study did not demonstrate an elevated false-alarm rate to the initial presentation of novel items
(22,
23). This was in large part due to a significantly more conservative response strategy (i.e., greater hesitancy to call an item old) in the schizophrenic subjects than in the comparison subjects, a pattern similar to that recently demonstrated by Danion et al.
(53). Given the discrepancies in the literature, the nature of response selection in schizophrenia, and its correlation with clinical features, may warrant further examination.
There are a number of limitations of this study that must be kept in mind while interpreting the results. First, all of the schizophrenic patients were taking psychotropic medications: differences in memory performance between these patients and the unmedicated healthy comparison group may be confounded by this factor. Prior work on the impact of medication status on episodic memory has been mixed, though most recent studies report no significant effect
(54,
55) or even slight cognitive benefit
(56–
58), particularly with atypical medications such as clozapine. Indeed, withdrawal of neuroleptic medication may even adversely affect memory performance
(59). A lack of a significant correlation between neuroleptic dose and measures of memory performance within the schizophrenic cohort also argues against a significant impact of this variable.
Second, although the comparison subjects did not differ from the patients in age or gender, they had a higher overall level of attained education and a greater level of mean parental education. Level of achieved education is generally not considered an adequate measure for comparison of overall intellectual capability. The lack of a significant correlation between mean parental education and memory performance suggests that this variable is not critical to explaining the false recognition results. Furthermore, several findings, including the ability to suppress false recognition after picture encoding, were based on within-group comparisons where there were no significant differences in age, education, mean parental education, or medication status.
Finally, we studied an all-male cohort, a decision based on the predominance of male schizophrenic patients at our affiliated clinic. While this strategy has the advantage of providing a more homogeneous study group, it may decrease the generalizability of our results. There are no a priori reasons to expect different results in female subjects, nor have there been gender differences detected in prior experiments using this paradigm.
In conclusion, recognition memory is impaired in patients with schizophrenia, but this impairment can be overcome, at least in part, when additional information is provided at the time of learning. This finding is relevant for the rehabilitation of schizophrenic patients by means of cognitive behavior interventions.