Several lines of evidence suggest that testosterone and other androgens might be effective in the treatment of major depressive disorder in certain men. First, depressed men in some studies
(1,
2) exhibited low plasma or serum testosterone levels, although this association is inconsistent and probably influenced by additional factors
(3–
5). Second, hypogonadal men often exhibit depressive symptoms, and testosterone replacement may improve these symptoms
(6,
7). This finding extends to men with HIV-induced hypogonadism
(8,
9). Third, anabolic steroid abusers, who ingest markedly supraphysiologic doses of testosterone and related androgens, sometimes develop manic or hypomanic symptoms during androgen use and depressive symptoms during androgen withdrawal
(10).
Fourth, uncontrolled studies of depressed men in the 1940s and 1950s
(10,
11), together with a few controlled studies in the 1970s and 1980s
(12,
13), have suggested that testosterone and other androgens might have antidepressant properties. Two more recent studies have produced somewhat contradictory results in this area. In the first, Seidman and Rabkin
(14) added open-label intramuscular testosterone enanthate, 400 mg every 2 weeks, to the existing antidepressant regimens of five men with refractory depression and low or borderline plasma total testosterone levels (200–350 ng/dl; reference range=300–990 ng/dl). These men’s mean score on the Hamilton Depression Rating Scale declined significantly over 8 weeks. Subsequently, Seidman et al.
(15) conducted a randomized, placebo-controlled trial of testosterone enanthate for 30 men with major depressive disorder and testosterone levels of 350 ng/dl or less. Unlike the men in the previous open-label study, however, these subjects were not simultaneously taking antidepressants. After 6 weeks of treatment, the investigators found no significant difference between testosterone and placebo in scores on the Hamilton Depression Rating Scale or Beck Depression Inventory.
The preceding lines of evidence, although inconsistent, suggest that testosterone might benefit at least some depressed men—perhaps especially those with low plasma testosterone levels—although these antidepressant effects are likely variable and idiosyncratic
(15). However, given the prevalence of treatment-resistant depression
(16), further investigation of testosterone’s possible antidepressant effects seems warranted.
Another stimulus for further investigation has been the introduction of new modes of administration for testosterone, such as patch
(17,
18) and gel
(19) preparations, which offer alternatives to intramuscular injections of testosterone and to potentially hepatotoxic orally active synthetic androgens
(20). We took advantage of one of these products, a transdermal 1% testosterone gel, to conduct a placebo-controlled, double-blind study of testosterone supplementation for men with refractory depression and low or borderline testosterone levels.
Method
Subjects
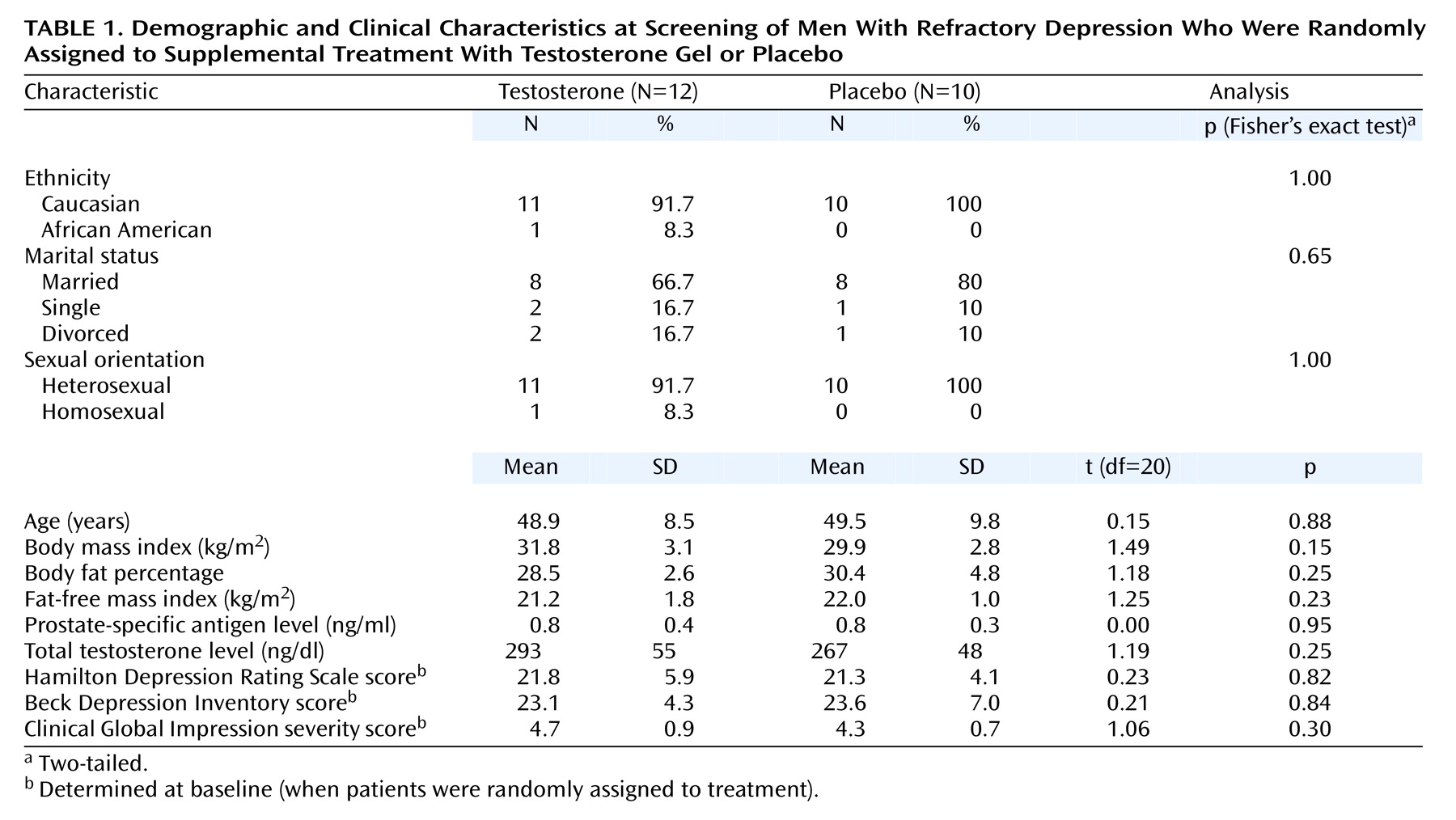
We recruited men meeting the following inclusion criteria: 1) age between 30 and 65 years, 2) current treatment with an adequate dose of antidepressant medication (as defined by the manufacturer’s published product information) for at least 4 weeks, 3) symptoms still meeting the DSM-IV criteria for current major depressive disorder, 4) a low or borderline morning serum total testosterone level (100–350 ng/dl; normal range in our laboratory, 270–1070 ng/dl), and 5) a normal prostate-specific antigen (PSA) level (<1.5 ng/ml in men aged 30–39, <2.5 ng/ml in men 40–49, <3.5 ng/ml in men 50–59, and <4.0 ng/ml in men 60–64).
Potential subjects, recruited through radio advertisements and referrals, were seen at the Biological Psychiatry Laboratory at McLean Hospital for an initial screening visit, scheduled before 10:00 a.m., when testosterone levels would normally be at their diurnal maximum
(21). After complete description of the study to the subjects, written informed consent was obtained. The subjects were then assessed with the depression module of the Structured Clinical Interview for DSM-IV (SCID)
(22) to confirm the diagnosis of current major depressive disorder. Subjects who did not meet the criteria for current major depressive disorder or who reported active suicidal ideation were excluded from the study. We next administered the American Urological Association Symptom Index for benign prostatic hyperplasia
(23), in which a score of 0–7 is defined as “mild,” 8–19 as “moderate,” and 20–35 as “severe.” Subjects scoring higher than 14 on this index were excluded. Blood was then collected for measurement of total testosterone and PSA levels.
Men displaying low testosterone and normal PSA levels according to our laboratory criteria were invited to return for a second screening evaluation. The assessments at this visit included 1) basic demographic questions, 2) the remainder of the SCID, 3) questions regarding history of previous antidepressant drug treatment, 4) the Hamilton Depression Rating Scale, 5) the Beck Depression Inventory, 6) the Clinical Global Impression, 7) a medical history, 8) a physical examination, including measurement of vital signs, calculation of body mass index, and digital rectal examination of the prostate, 9) collection of blood and urine samples for urinalysis, standard chemistries, blood cell counts, and HIV serology measurements, 10) ECG, and 11) determination of body fat with calipers, together with calculated fat-free mass index, a measure of muscularity previously developed in our laboratory
(24). Subjects were excluded if they exhibited 1) any substance use disorder within the past year (or illicit anabolic steroid use at any time in their lives), 2) current or past psychotic symptoms, 3) a history of bipolar I or bipolar II disorder, 4) any abnormality during the digital rectal examination, or 5) evidence of other clinically significant medical disease. The qualifying subjects then began 1 week of single-blind administration of placebo gel. All subjects continued taking their existing antidepressant medications, together with any other prescribed medications, at their present doses throughout the study.
Week 0 (Baseline)
After the single-blind placebo period, the subjects returned for a baseline visit, during which they were assessed for scores on the Hamilton Depression Rating Scale, Beck Depression Inventory, and Clinical Global Impression measure of severity of illness; for adverse events; and for vital signs. Results from the ECGs and the laboratory tests for which blood was drawn at screening were also reviewed. Subjects were withdrawn from the study at this point if they 1) displayed more than 50% improvement on the Hamilton Depression Rating Scale or Beck Depression Inventory after the placebo treatment or 2) were found to have a clinically significant abnormality on the laboratory tests or ECG. The subjects who qualified to continue in the study were then randomly assigned to receive either 10 g of 1% testosterone gel or placebo gel daily for the next 7 days. Drug and placebo were supplied in identical-appearing packets containing either 2.5 g of testosterone gel or a placebo gel, so that the starting dose was four packets per day.
Randomization and Blinding
An independent research assistant, not otherwise involved in the study, placed testosterone and placebo gel packets in numbered containers, using a randomization schedule that was not “blocked” or stratified. Thus, each subject had a 50% chance of receiving either treatment, determined independently of the treatment assignment of all other subjects. At baseline, the principal investigator (H.G.P.) assigned sequential numbers to the subjects as they were randomly assigned and supplied study medication to them from the corresponding numbered containers. Each subject and all study investigators (except for the unblinded dose adjuster; see following section) remained blinded to that subject’s treatment assignment until all of his ratings were completed at study termination.
Week 1
At week 1, the subjects were again assessed for scores on the Hamilton Depression Rating Scale, Beck Depression Inventory, and Clinical Global Impression (both severity of illness and improvement from baseline); for adverse events; and for vital signs. In addition, the subjects provided blood for determination of total testosterone level; the blood sample was drawn at least 4 hours after the morning application of the gel. This level was communicated to a separate unblinded investigator (J.I.H.), who ordered a reduced dose of gel (7.5 g, or three packets) for any subject exceeding the normal range (>1070 ng/dl). This investigator also performed sham dose adjustments for the placebo-treated patients to ensure that the subjects and investigators remained blinded. The unblinded dose adjuster was permitted only to lower, not to raise, the dose of study drug. Throughout the study he had no contact with any of the subjects and no discussions with the other investigators regarding the subjects.
Weeks 2, 4, 6, and 8
The subjects were seen at each of these time points for assessment with the Hamilton Depression Rating Scale, Beck Depression Inventory, and Clinical Global Impression; for determination of adverse events; and for measurement of vital signs. At week 8, the subjects also received an additional determination of PSA level and measurement of weight and body fat. When these assessments were completed, the blind was then broken and the treatment assignment determined. Since randomization was performed without blocking or stratification, as already mentioned, this unblinding gave the investigators no information regarding the treatment assignment of subsequent subjects still undergoing blinded treatment. Subjects who had received testosterone and who felt that they had responded were referred to their original physicians for consideration of continued testosterone treatment. Subjects who had received placebo were offered up to 4 weeks of treatment with testosterone gel by the investigators, followed by referral to their original physicians.
Subjects were withdrawn before the 8-week point if they 1) voluntarily elected to withdraw for any reason, 2) displayed an adverse event judged clinically significant by the investigators, or 3) failed to comply with the requirements of the protocol.
Objectives, Outcomes, and Group Size
We hypothesized that testosterone supplementation would be more effective than placebo in reducing depression, as assessed by improvement in scores on the Hamilton Depression Rating Scale, Beck Depression Inventory, and Clinical Global Impression. We also hypothesized that the subjects receiving testosterone gel would exhibit greater reductions in body fat and increases in muscle mass than the subjects receiving placebo. The study was planned to be exploratory and to guide future studies by providing an estimate of the magnitude and standard deviation of the changes in outcome measures for each group. We chose a group size of 22 for this purpose, recognizing that this small study group would limit our power to detect significant differences between groups in outcome measures.
Statistical Analysis
The baseline characteristics of the two groups were compared by using Fisher’s exact test for categorical variables and the t test for continuous variables. Outcomes were assessed in both an intent-to-treat group of patients, including those with at least one available score on the efficacy measures, and a completers group, defined as patients who completed the 8-week treatment period.
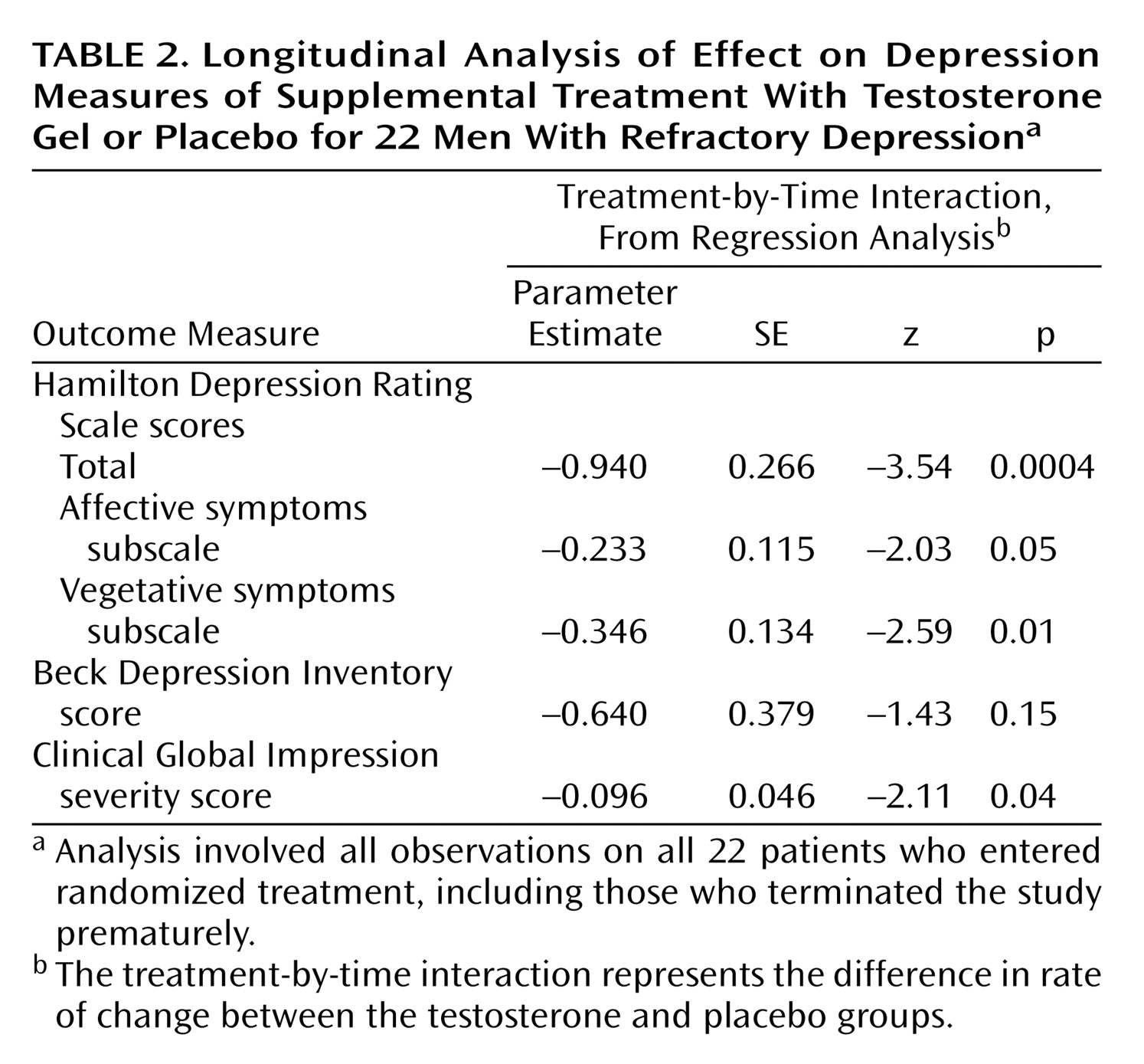
The primary protocol-defined analysis of efficacy was a repeated measures random regression analysis comparing the two groups’ rates of change in scores on the Hamilton Depression Rating Scale, Beck Depression Inventory, and Clinical Global Impression severity measure during treatment, conducted according to methods previously described
(25,
26). Our model for the mean value for each outcome measure included terms for treatment, time, and treatment-by-time interaction. We modeled time as a continuous variable, with weeks ranging from 0 to 8. The measure of effect was the treatment-by-time interaction, which can be interpreted as the difference in slope, or rate of change per unit of time, of the efficacy measure. To account for the correlation of observations within individuals, we calculated the standard errors of the parameter estimates using generalized estimating equations, with compound symmetry as the working covariance structure, as implemented by the PROC GENMOD command in SAS software (Cary, N.C., SAS Institute).
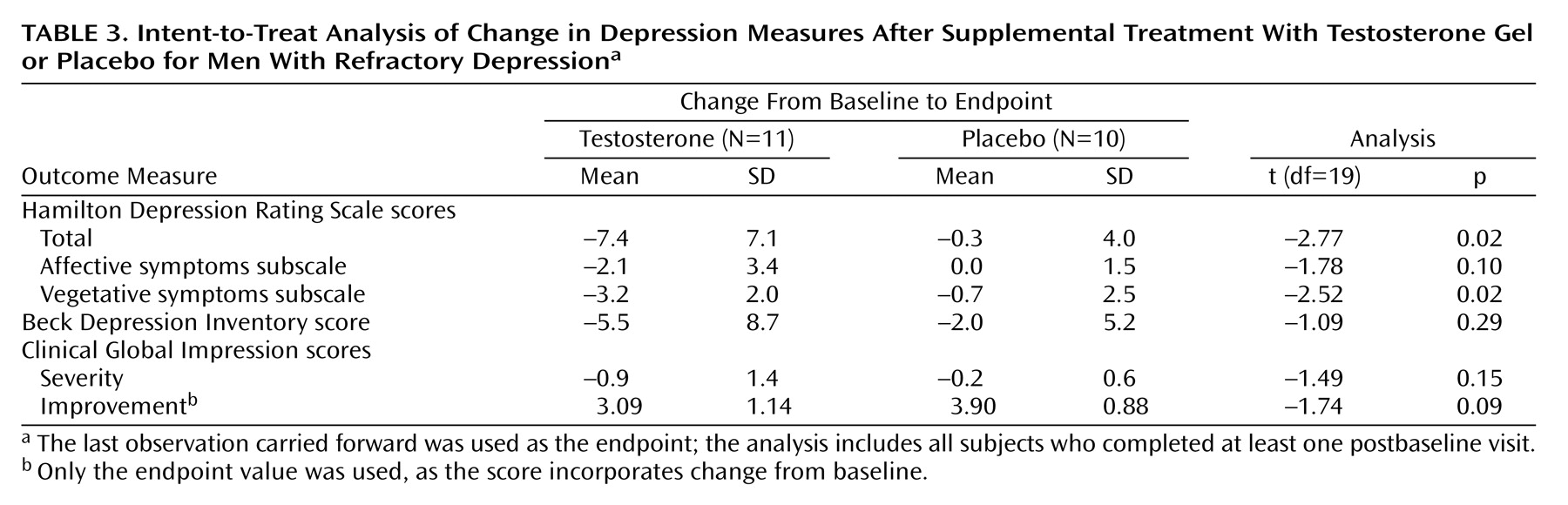
In the secondary intent-to-treat analysis, we used the last observation carried forward for all subjects completing at least one postbaseline assessment and conducted t tests to compare the groups on change from baseline to endpoint in scores on the Hamilton Depression Rating Scale, Beck Depression Inventory, and Clinical Global Impression severity scale.
For laboratory measures, including body fat and fat-free mass index, we computed the mean difference between the endpoint and baseline measurements, and we then compared the treatment groups using t tests. We calculated correlation coefficients by using rank-transformed data (Spearman rank correlation). All statistical tests were two-sided with alpha=0.05.
Discussion
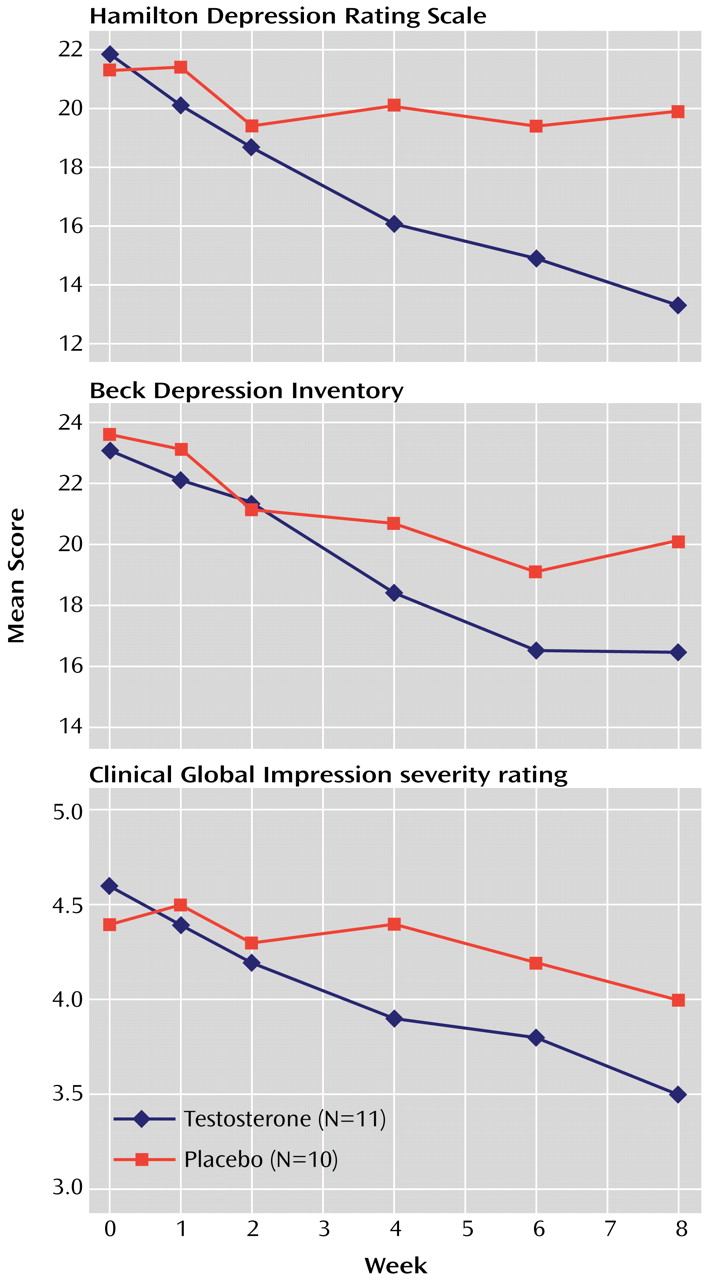
We conducted an 8-week randomized, placebo-controlled trial of testosterone transdermal gel with 22 men who had treatment-resistant depression and low or borderline serum total testosterone levels (≤350 ng/dl). Testosterone gel, added to the subjects’ existing antidepressant regimens, proved significantly superior to placebo in reducing scores on the Hamilton Depression Rating Scale and the Clinical Global Impression severity scale, although not on the Beck Depression Inventory. These findings suggest that testosterone supplementation may produce antidepressant effects in some men with treatment-resistant depression.
Two observations in the study deserve particular comment. First, the men in our study, with a mean age of only 46.9 years, displayed a mean total testosterone level of only 403 ng/dl (SD=152)—strikingly lower than the mean of 605 ng/dl (SD=212) among men aged 45–54 in a typical study of the general population
(27), and even slightly below the mean of 429 (SD=124) in a recent study
(4) of men with major depressive disorder in their 60s and 70s, when testosterone would normally be much lower than in middle age. These observations suggest that low testosterone levels may be unexpectedly common in middle-aged men with treatment-resistant major depressive disorder, perhaps because chronic depressive symptoms lead to blunting of the hypothalamic-pituitary-gonadal axis
(4) or, possibly, because of effects of the antidepressant medications themselves.
Second, testosterone gel appeared to benefit psychological aspects of depression (represented by the depressed mood, guilt, and psychological anxiety items of the Hamilton Depression Rating Scale) to nearly the same degree as the somatic aspects of depression (represented by the Hamilton scale items involving sleep, appetite, libido, and somatic symptoms). This finding differs somewhat from that of a previous study of testosterone for HIV-infected men
(8), who experienced substantially greater improvement in vegetative than in affective symptoms—raising the important question of whether testosterone benefited their depression only because of its effects on somatic symptoms such as fatigue and libido
(28). One reason for this difference in findings may be that HIV-infected men have higher initial levels of somatic symptoms, such as anorexia, weight loss, and fatigue, than did our study group of medically healthy depressed men.
The mechanism of testosterone’s antidepressant effects, and the variability of these effects among subjects, remains speculative. Testosterone modulates brain monoamine levels, as evidenced by one study
(29) that showed lowered CSF 3-methoxy-4-hydroxyphenylglycol and elevated 5-hydroxyindoleacetic acid (5-HIAA) levels in normal volunteers after methyltestosterone administration. Notably, CSF 5-HIAA changes were positively correlated with activation scores in this study, suggesting that the psychiatric effects of testosterone might be mediated through serotonergic function. The inconsistent relationship between endogenous plasma testosterone levels and depression, and the inconsistent antidepressant effects of exogenous testosterone, may be attributable to the weak association between plasma and CSF testosterone levels. For instance, in one recent study
(30), patients with posttraumatic stress disorder had low CSF testosterone levels but normal plasma testosterone levels, and there was no significant association between plasma and CSF testosterone levels.
Several limitations of our study must be considered. First, the small number of subjects limits statistical power. Second, the study assessed the effects of testosterone for only 8 weeks and does not permit conclusions about long-term efficacy or safety for depressed men. Third, the blind was broken for each subject individually at termination, rather than for all subjects at the conclusion of the study, as is more conventional. However, since randomization was performed without blocking or stratification, investigator bias due to unblinding should be largely eliminated, as we have already explained. Fourth, the study used a single serum total testosterone level to determine eligibility. More sophisticated laboratory screening (including measurements of free testosterone, sex hormone-binding globulin, and pituitary gonadotropins, together with serial chemistry and hematology measurements) might help to identify candidates for treatment, laboratory predictors of treatment response, and potential adverse effects. Fifth, testosterone was added to existing antidepressant treatment for men who were not responding to antidepressants. It is unclear whether testosterone would prove equally effective for depressed men not currently taking antidepressants. The findings of Seidman and colleagues
(14,
15), summarized earlier in this article, combined with the results of the present study, suggest that testosterone might be more useful as a supplement to conventional antidepressant treatment than as an antidepressant by itself. Sixth, our study was limited to men because of the masculinizing effects of testosterone. However, preliminary data
(31) suggest that in much lower doses, testosterone may exhibit antidepressant effects in women as well; this possibility deserves exploration.
The benefits of testosterone supplementation must also be weighed against potential risks. For example, in one early paper
(32) it was reported that of five men receiving imipramine for depression, four developed paranoid symptoms when the synthetic androgen methyltestosterone was added to their regimen. In our study, by contrast, no subject assigned to testosterone was rated on any study visit as having “ideas of reference” or “delusions of reference and persecution” on the “paranoid symptoms” item of the Hamilton Depression Rating Scale. However, none of our subjects was receiving a tricyclic antidepressant.
Our brief study also did not permit assessment of possible longer-term risks of testosterone administration, such as gynecomastia, adverse effects on lipid fractions, or gradual exacerbation of benign prostatic hyperplasia
(33). Long-term exogenous testosterone administration also suppresses the hypothalamic-pituitary-testicular axis, with consequent oligospermia and the risk of severe temporary suppression of gonadal function if testosterone is abruptly withdrawn
(33). Finally, there remains the question of whether long-term testosterone supplementation might increase the risk of prostate or other cancers. The present data on this issue are still inadequate
(28,
33,
34).
In conclusion, these findings may have important consequences for public health. In a given year, about 8% of American men over the age of 30 years exhibit major depressive disorder
(35), and many of these cases will be partially or completely refractory to an adequate trial of antidepressant medication
(16). If this subgroup with refractory depression has a 43% prevalence of low testosterone levels, as found in the present study, then hundreds of thousands of men in a given year might at least theoretically be candidates for testosterone supplementation to treat depression. Given the size of this population, together with the increased availability and convenience of transdermal testosterone preparations, it seems important to assess carefully both the benefits and the risks of this antidepressant treatment strategy.