Several brain imaging studies have previously linked components of emotional behaviors to limbic and paralimbic structures (insula, amygdala, medial temporal cortex, anterior cingulate) and to subcortical regions (thalamus, caudate, hypothalamus), as well as to medial, dorsolateral, and orbitofrontal regions
(2–
4). An unresolved question is whether the same neural structures mediate both positive and negative emotions. Some studies have supported the hypothesis that positive and negative emotions are processed in different neural structures
(2,
3). Davidson and Irwin
(5) proposed that the right prefrontal cortex is more active during negative emotions such as fear, disgust, or sadness than during positive emotions. Other authors have suggested a nonlateralized prefrontal pattern in negative and positive emotion in which ventral areas are active during negative emotion and dorsal areas are active during positive emotion
(6,
7). In addition, while some studies
(8,
9) have reported that the anterior cingulate and medial prefrontal cortex play a specific role in the cognitive processing of emotional stimuli, little is known about the neural basis of self-referential processing of these types of stimuli.
The aim of the current study was to identify the brain regions mediating interactions between self-related processing and emotional processing. The experiment was designed to build on previous work demonstrating that words processed with reference to the self (some studies used nouns, not all of which had emotional valence) are generally better remembered than material processed in semantic terms
(12). Numerous studies have established the reliability and validity of this phenomenon, labeled the “self-reference effect”
(12). Paradigms that use emotional words to examine the self-referential effect allow assessment of self-related processes within an emotional context. Craik et al.
(13), using a self-reference effect paradigm and positron emission tomography (PET), determined that self-encoding of adjectives describing personality traits yielded specific activation of the right dorsomedial prefrontal cortex. However, this study did not allow for comparison between stimuli with positive and negative emotional valences and therefore did not provide a basis for investigating the emotional bias toward negative stimuli in depression.
Method
Subjects
Fourteen healthy subjects (eight women, six men; mean age=26.4 years, SD=4.5, range=20–36), screened for absence of psychiatric and neurological disorders with a clinical interview, were recruited from the University of Toronto community. At the time of initial screening, participants’ mood was assessed with the Beck Depression Inventory
(14). All subjects were fluent in English, were right-handed as judged by the Edinburgh Handedness Inventory
(15), and had normal or corrected-to-normal visual acuity. After a complete description of the study to the subjects, written informed consent was obtained. The study was approved by research ethics boards at Baycrest Centre, Sunnybrook and Women’s College Health Sciences Centre, and the University of Toronto. Subjects were reimbursed for their participation and time. Four subjects whose fMRI time series had perceptible, residual head movements greater than 1 mm were excluded from further analysis. The remaining 10 subjects (seven women, three men; mean age=25.8 years, SD=4.4; mean number of years of education=17.9, SD=2.0; mean Beck Depression Inventory score=2.4, SD=2.9) were included in the study.
Verbal Stimuli
Thirteen lists of 10 personality-trait adjectives were constructed from Anderson’s list of personality-trait words
(16). The lists were used in the judgment tasks described in the next section (additional lists were used in a subsequent recognition test that will be detailed elsewhere, in a separate report). Within each of the 13 lists, half of the words were unambiguously positive and half were negative, selected from the top 20% and bottom 20%, respectively, of Anderson’s sample. One list was used for practice trials. The other 12 lists were shown to the participants during the scan acquisitions. The study included three different judgment conditions: self-referential processing, other-referential processing, and letter recognition (used as the baseline control task). Four lists were assigned to each of three judgment conditions. Each positive and negative word occurred in only one of the lists, and each word was randomly assigned to only one of the three judgment conditions for each subject. Therefore, each subject received a unique protocol.
Task Design
The fMRI scans were acquired while patients performed the judgment tasks. Stimuli were generated by a personal computer with SuperLab Pro software (Cedrus Corp., San Pedro, Calif.) and appeared on a back-projection screen mounted outside the scanner bore; subjects viewed the screen using angled mirrors.
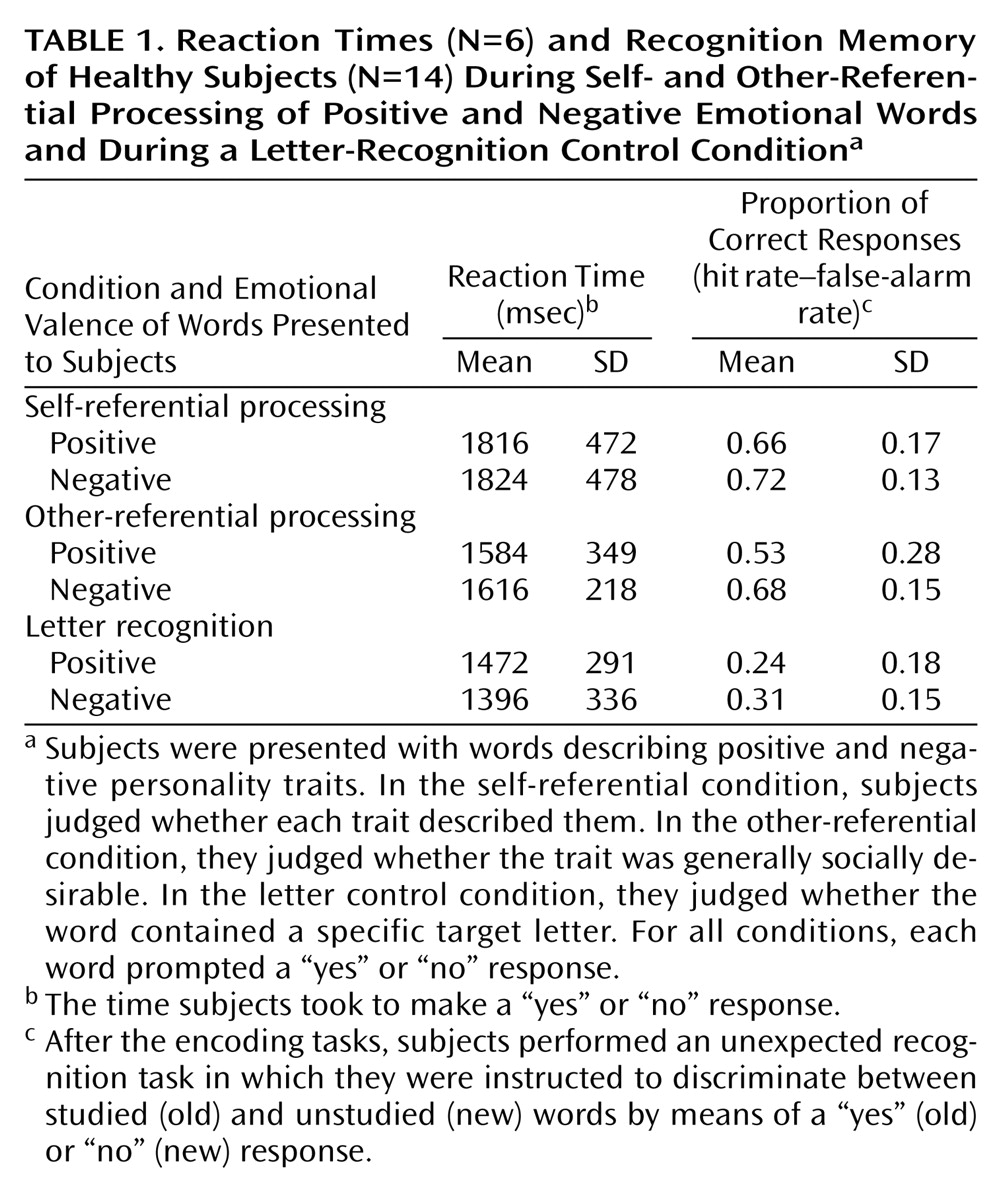
Depending on instructions presented before the start of each set, participants made one of three different judgments about the presented words. Subjects were not required to directly rate the valence itself. In the self-referential condition, subjects judged whether each trait described them. In the other-referential condition, participants judged whether the trait was socially desirable. In the letter-recognition control condition, participants judged whether the word contained a specific target letter (i.e., “e” or “a”; a different letter was used for each list). For all conditions, each word prompted a “yes” or “no” response, and subjects pressed a button with the right index or middle finger, respectively, to indicate their decision. Button presses were recorded by using an MRI-compatible keypad (Lumitouch, Lightwave Technologies, Richmond, B.C., Canada). Participants were not explicitly asked to remember the words.
Subjects performed each judgment condition four times across three scanning runs. At the onset of each condition, an instruction cue was presented for 9 seconds (e.g., “task: self condition”). Each condition contained trials consisting of two word types: negative words and positive words. Negative and positive words were randomly intermixed and separated by a fixation crosshair displayed on the center of the screen. Item order was counterbalanced such that positive and negative items followed each other equally often. Each list of adjectives constituted 10 trials. Each trial consisted of a fixation crosshair displayed for 500 msec followed by an adjective displayed for 4500 msec, then another fixation point displayed for 5000 msec. Participants responded by pressing a keypad at any time between the onset of the stimulus and the onset of the 500-msec fixation of the next trial. To control for order effects, blocks within a run (40 words per run, 10 words per block; a total of four blocks per run) were presented in random order, with no two consecutive blocks in the same condition. Both response and reaction times were recorded by SuperLab.
Encoding tasks were immediately followed by an unexpected recognition task. For the recognition task, subjects were instructed to discriminate between studied (old) words and unstudied (new) words. Each word prompted a “yes” (old) or “no” (new) response, and subjects pressed a button with the right index or middle finger, respectively, to indicate their decision.
Functional Imaging
Imaging was performed at Sunnybrook and Women’s College Health Sciences Centre by using a 1.5-T Signa MRI system (CV/i hardware, LX8.3 software; General Electric Medical Systems, Waukesha, Wis.) with a standard quadrature birdcage head coil. Structural magnetic resonance images were acquired by using a three-dimensional T
1-weighted spoiled gradient recall acquisition sequence (TR=12.4 msec, TE=5.4 msec, flip angle 35°, 22×16.5 field of view, 256×196 acquisition matrix, 124 axial slices, 1.4 mm thickness). Functional images were collected by using a T
2*-weighted pulse sequence with spiral k-space readout, offline gridding, and reconstruction (TE=40 msec, TR=2000 msec, flip angle=80°, 90×90 effective acquisition matrix, 20 cm field of view, 24 axial slices, 5 mm thickness) optimized for sensitivity to blood-oxygenation-level-dependent (BOLD) signal contrast
(17). Functional data were acquired in three separate runs, each lasting 7 minutes and 56 seconds (including an initial 40 seconds for machine equilibration).
Statistical Analysis
Analysis of fMRI data
Images of brain activation were computed and overlaid on anatomic images by using the Analysis of Functional NeuroImaging (AFNI) program
(18). Time series data were first spatially coregistered to correct for head motion by using a three-dimensional Fourier transform interpolation and were then detrended to a constant reference scan by using a fifth-order polynomial. The data were subjected to two analyses: one to examine the effects of self-processing regardless of word valence, and the other to clarify the role of valence.
Analysis of hemodynamic response
Percent changes in signal intensity during the self-referential and other-referential conditions with respect to the letter-recognition control condition were analyzed by using voxelwise correlations of the self-referential and other-referential time series with square-wave reference vectors
(19) shifted to account for the delay in hemodynamic response. This method produced two activation images per participant: one for the self-referential condition versus the letter-recognition control condition, and one for the other-referential condition versus the letter-recognition control condition. These activation images were then transformed into Talairach coordinates
(18,
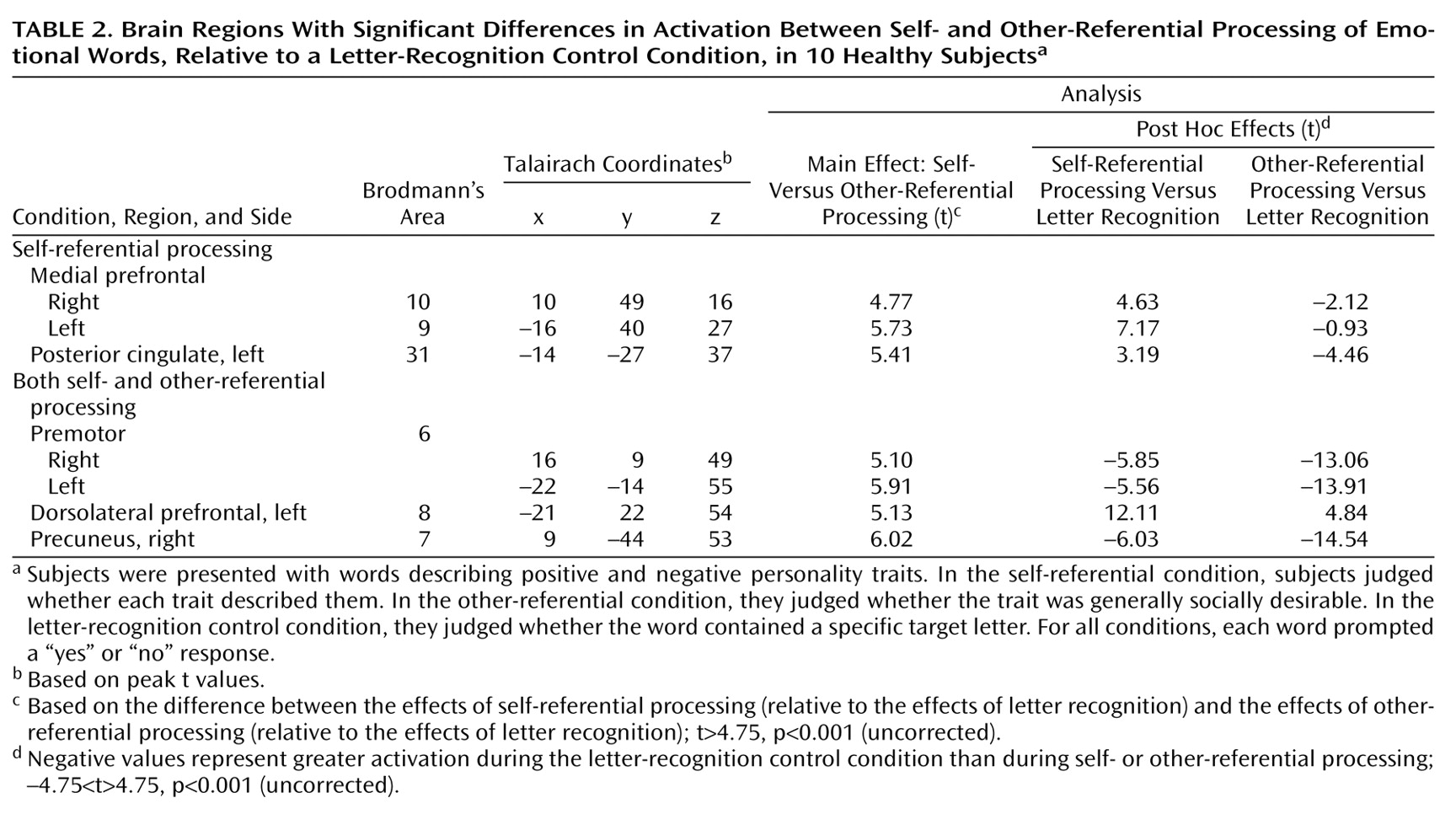
20) and smoothed by using a Gaussian filter with a 6-mm full width at half maximum to increase the signal-to-noise ratio. The latter step was performed to facilitate the subsequent group analysis consisting of a mixed-effect, voxelwise two-factor analysis of variance (ANOVA) of the percent changes in signal intensity, with judgment condition (self- or other-referential) as the within-subjects factor and subject as a random factor. Because of our a priori hypothesis targeting the medial prefrontal region, the statistical cutoff for the main comparison between the self and other conditions was set at p<0.001, uncorrected.
Analysis of valence effect
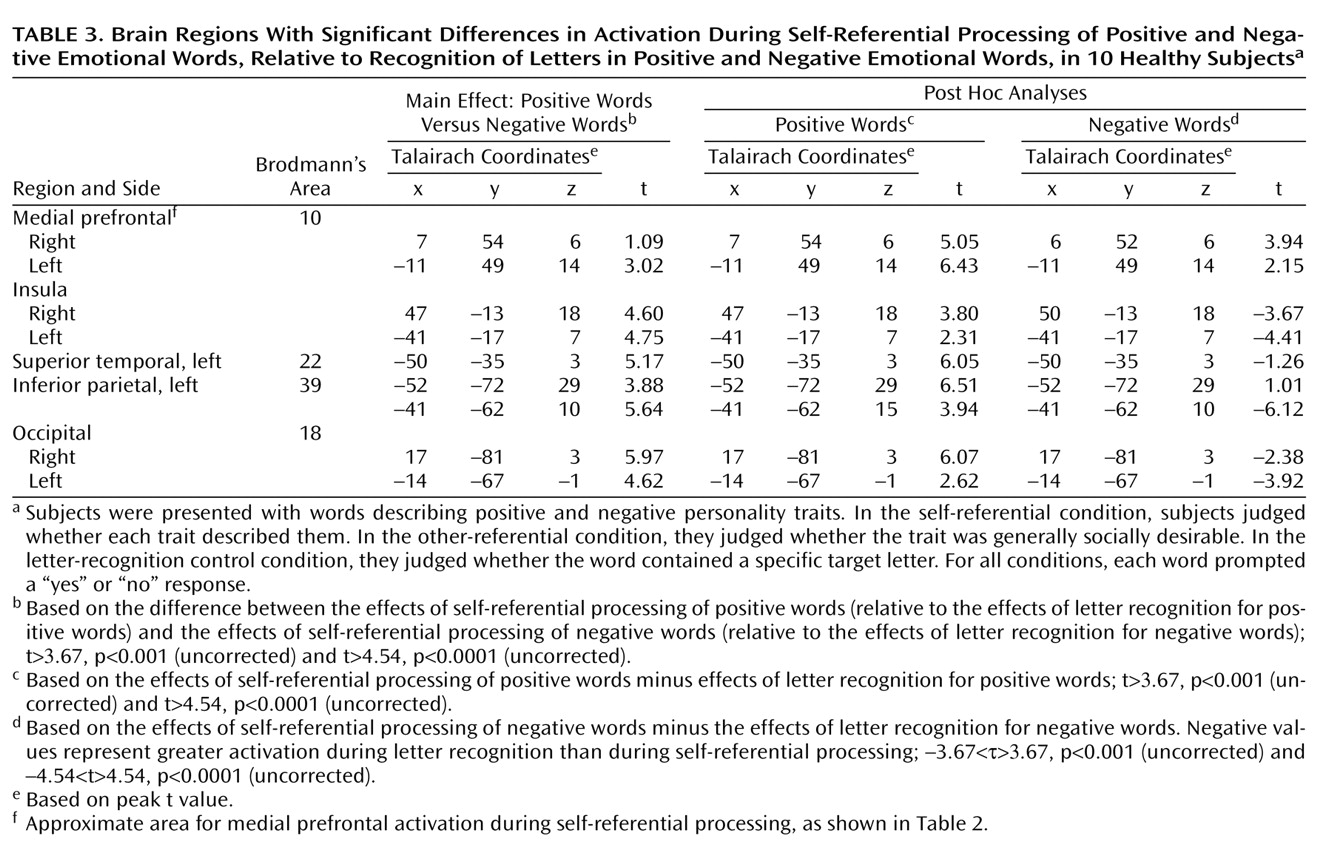
In the second analysis, we contrasted positive and negative words to determine the effect of valence in self-referential processing. To this end, we compared responses to positive and negative words in the self-referential condition relative to the letter-recognition control condition. AFNI was used to deconvolve the hemodynamic response function on a voxelwise basis from the time series data to interpret the activations associated with positive and negative words randomly presented within a given block. The best linear least-squares fit was calculated for the following model parameters: constant baseline, linear trend in time series, and BOLD response deviation from baseline for each condition (self-referential processing of positive words and self-referential processing of negative words). This fit of the parameters produced an estimate of the hemodynamic response for the image 0–5 TR (10 seconds) after the stimulus onset for each condition relative to the control condition. Subsequently, activation images were created corresponding to 3 TR after the stimulus onset for each of the conditions contrasted with the corresponding letter-recognition condition (self-referential processing of positive words versus letter recognition for positive words, self-referential processing of negative words versus letter recognition for negative words). The images were then transformed into Talairach coordinates and spatially smoothed as reported earlier.
The subsequent group analysis consisted of a mixed-effect, voxelwise two-factor ANOVA with condition (self-referential processing of positive words, self-referential processing of negative words) as the within-subject factor and subject as a random factor. The statistical cutoff for the main comparison of self-referential processing of positive and negative words was set at p<0.001, uncorrected. Post hoc specific contrast manipulations were then investigated in more detail.
In addition, because both the self-referential and the letter-recognition conditions involved both explicit and implicit processing of the emotional aspects of the words, it was also important to determine whether the comparison of self-referential processing with letter recognition removed a specific valence effect. This possibility was assessed by contrasting activation associated with positive and negative words across all the different conditions.
Discussion
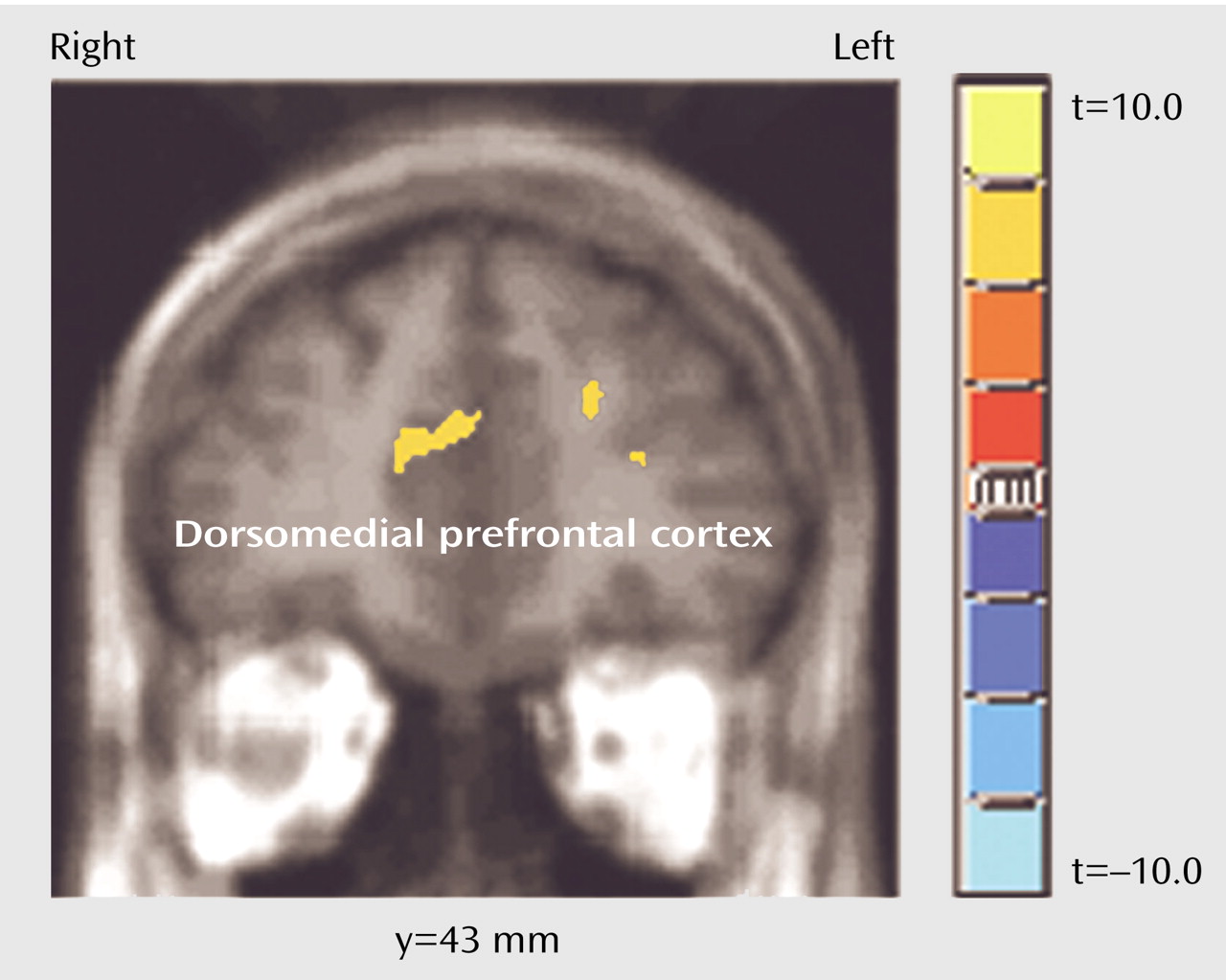
The goal of this study was to identify brain regions mediating self-referential processing of positive and negative emotional words. The main finding was that emotional stimuli processed in a personal context were associated with a different pattern of brain activations than were identical stimuli considered in a general context. Self-referential processing of emotional words induced a unique activation in the dorsomedial prefrontal cortex. Moreover, the activation seen in the dorsomedial prefrontal cortex, especially on the right, was present irrespective of the valence of the words, suggesting that this region is involved in self-referential processing of all emotional stimuli, regardless of emotional valence.
The fMRI results additionally showed that the magnitude of the signal in the self-referential condition was greater than in the other-referential condition in left dorsomedial prefrontal cortex regions (Brodmann’s area 8/9), consistent with our earlier study
(13) and a large literature on semantic processing of words and the hemispheric encoding retrieval asymmetry (HERA) model
(21). Likewise, the association of self-reference with posterior cingulate activity replicates earlier findings implicating the posterior cingulate in aspects of emotional processing
(22).
One possible explanation for the selective right dorsomedial prefrontal cortex activity might be the targeted use of strictly emotional words. However, processing of emotional words is not consistently associated with activations of the dorsomedial prefrontal cortex across brain imaging studies. One PET study
(23) found increased activity in the medial prefrontal regions when subjects passively viewed emotional words relative to passively viewing animal names, while another study
(24) showed brain activation mainly in the retrosplenial cortex associated with threat-related words and no activation in the dorsomedial prefrontal cortex.
We suggest that the dorsomedial prefrontal cortex activity is not likely to be related to emotional content per se but is due to the self-referential processing of the emotional stimuli. Several converging lines of evidence support this interpretation. The right dorsomedial prefrontal cortex has been reliably activated across a wide range of emotional tasks with or without verbal materials, such as recollection of personally affect-laden life events
(25), attention to subjective feeling
(8), and processing of emotion-related meanings
(7). All these tasks can be characterized as requiring access to, or manipulation of, explicit representation of different aspects of the self and integration of these aspects with emotional reactions and experience.
The new finding from this study is that the right dorsomedial prefrontal cortex is activated during self-evaluation regardless of whether the valence of the words is positive or negative. Unlike other works showing hemispheric asymmetry in the mediation of positive and negative emotions
(5), we found no evidence for laterality effects due to the valence of the emotional words.
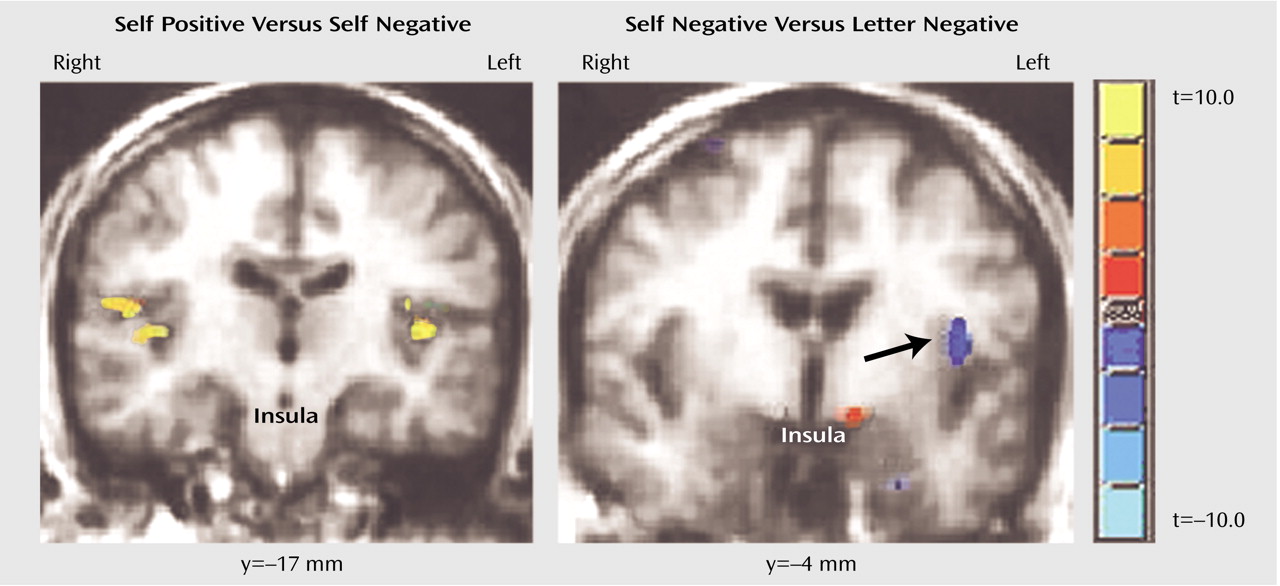
A further finding is the difference in regions outside of the prefrontal cortex that were identified with respect to the self-referential processing of positive and negative emotional words. The self-referential processing of negative words induced significant BOLD signal reduction in the right inferior parietal lobe, the left and right insula, and more posterior regions. One interpretation is that the processing of negative words in the self-referential condition necessitated more cognitive effort than the processing of positive words. This interpretation would be consistent with recent suggestions by Pochon et al.
(26) of a negative modulation of limbic activity by task complexity.
The insula and parietal regions are also associated with anxiety, depression, and provocation of feelings of sadness
(27), suggesting that these regions are more generally involved in the integration of internal states. The present experiment was not designed to elicit explicit emotional feeling states in subjects. However, to avoid feeling emotions and maintain a self-protective mode, subjects may have attempted to inhibit their emotional responses during self-processing of negative words
(28).
The dorsomedial prefrontal cortex and the adjacent anterior cingulate have direct reciprocal connections with the inferior parietal cortex, dorsolateral prefrontal cortex, and posterior cingulate. The dorsomedial prefrontal cortex and the dorsal anterior cingulate also communicate with the paralimbic structures, the ventromedial prefrontal cortex, and brainstem regions indirectly through the rostral and subgenual cingulate areas
(29). Therefore, by virtue of the capacity to receive inputs from these limbic and paralimbic regions and to project to other prefrontal areas, the dorsomedial prefrontal cortex is a suitable region for integrating cognitive processing with emotional reactions and experience.
The human “self model” is a theoretical construct comprising essential features such as feelings of continuity and unity, experience of agency, and experience of a body-centered perspective
(30). We propose that one specific role of the right dorsomedial prefrontal cortex is to represent states of an emotional episodic “self” and then to process emotional stimuli with a personally relevant perspective. This proposition is in line with studies showing activations within both the left and right dorsomedial prefrontal cortex during “theory of mind” tasks
(31). Because emotions generally signal issues related to the self, subjects may use emotional cues during some theory of mind tasks to differentiate self from other; this self-related emotional processing is indicated by an increase of activity in the right dorsomedial prefrontal cortex.
This study had some limitations that must be considered in interpreting the findings. Owing to technical difficulties, only 10 of the original 14 subjects were included in the final analysis. Thus, the study group size may have been underpowered to detect a valence effect. Further studies with larger numbers of subjects are therefore needed to confirm the results.
To assess self-processing within an emotional context, we used an incidental encoding task in which subjects made different evaluative judgments about emotional words. Behavioral results showed that response times for judging each word were similar in the self- and other-referential conditions. However, data on response reaction times were available for only six subjects. Therefore, we cannot definitely rule out the possibility that, because of the small number of subjects, the differences in reaction times may actually reflect real differences in brain activation between the self- and other-referential conditions.
Positive and negative emotional words were not matched for the arousal associated with them. Negative stimuli are typically more arousing than positive words, and fMRI differences between the word types may be due to this potential confound. However, we found that positive words induced greater activation than negative words. Moreover, self-referential processing of negative words was associated with BOLD reductions in several regions, an unlikely effect of emotional arousal, as suggested by past observations
(32).
To replicate our previous PET findings
(13), we used the same paradigm with emotional words for each condition. Further studies including neutral words may help to confirm our findings on the role of the dorsomedial prefrontal cortex in self-referential processing of emotional stimuli with different valences.
In summary, our findings provide support for the involvement of a widely distributed set of brain regions in the processing of self-referential information. Among these regions, the right dorsomedial prefrontal cortex appears most specific for the more subjective, perspective-taking aspects involved in emotional evaluation. The common role of the dorsomedial prefrontal cortex in evaluating both positive and negative stimuli further suggests that the dysfunction of the dorsomedial prefrontal cortex frequently reported in mood disorders
(33) may subserve the bias of emotional processing in depression.