Despite this interest, to our knowledge grief has not been the subject of previous neuroimaging research. Findings from several related phenomena, however, provide direction regarding the possible neural correlates of grief. Recent functional imaging research has revealed that during the successful retrieval of autobiographical memories elicited by name-cued recall of family members and friends, the strongest and most consistent activation is in the caudal portion of the left posterior cingulate cortex
(5). Perception of familiar faces and voices also is associated with increased neural activity in the posterior cingulate cortex, including the retrosplenial cortex
(6). Transient sadness induced in a variety of ways has been associated with increased activity in the subgenual cingulate (Brodmann’s area 25) as well as the anterior insular cortex and prefrontal cortex
(7,
8). In addition to other emotions evoked within a single positron emission tomography study, sadness induced by the free recall of a close relative’s or friend’s death was associated with activation of the bilateral insula, anterior cingulate cortex, orbitofrontal cortex, basal forebrain, caudate nucleus, lenticular nucleus, left thalamus, midline cerebellum, and anterior and dorsal pons
(8).
We therefore predicted that in bereaved participants, the induction of transient grief would be associated with activity in the posterior cingulate cortex, anterior cingulate cortex, and prefrontal cortex. Given that grief is a complex cognitive, emotional, and physiological state, we anticipated that grief would be mediated by a distributed network of structures that would include, but not be restricted to, these areas.
In order to elicit grief, we used a 2×2 (person-by-word) factorial design, creating four conditions. Each of the four conditions consisted of 15 picture-word composites. By comparing a picture of the deceased with a picture of a stranger (person factor) and the personalized grief-related words with matched neutral words (word factor), we were able to elicit grief through two distinct methods in bereaved subjects.
Results
The mean duration of bereavement was 8.5 months (range=1–12). The participants were Caucasian (N=7) and Asian American (N=1). The deceased relatives were mother (N=4), father (N=3), and husband (N=1). The mean age was 41.9 years (range=19–58). All subjects reported that they were able to clearly see all stimuli.
The 60 skin conductance responses (obtained during scanning) and subjective ratings (obtained immediately after scanning) for each participant were transformed into z scores for this analysis, in order to determine the individual pattern of responses to the pictures across conditions, rather than the absolute magnitude of change. This rescaling allowed analyses of each individual’s responses according to her own average magnitude of response and degree of variability across trials.
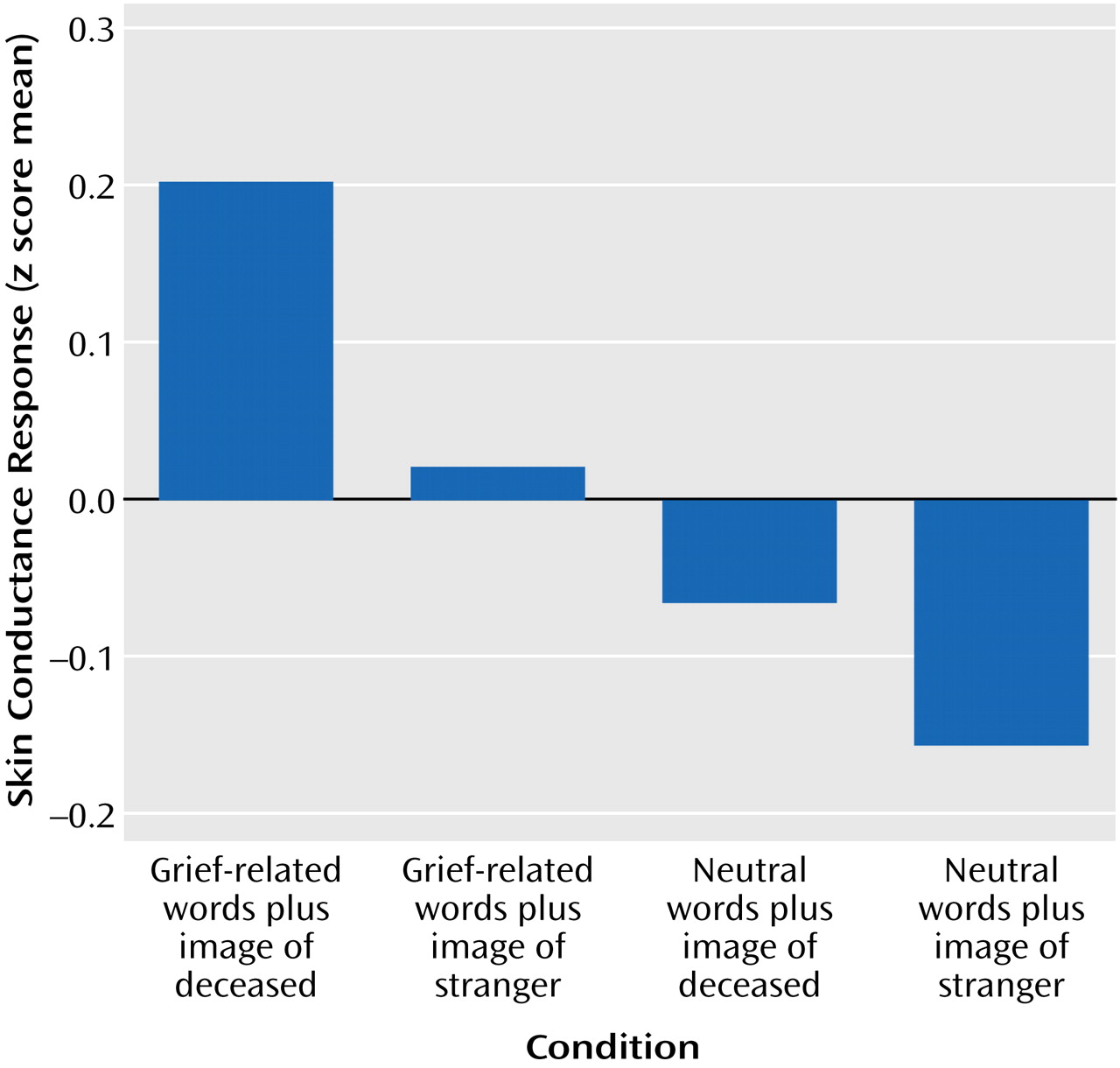
Differences in physiological arousal among the conditions were indicated in the electrodermal responses, which are primarily an index of sympathetic nervous system activity. There was a significant difference among skin conductance responses (
Figure 1), indicating that the grief-related stimuli elicited stronger electrodermal responses than the non-grief-related stimuli.
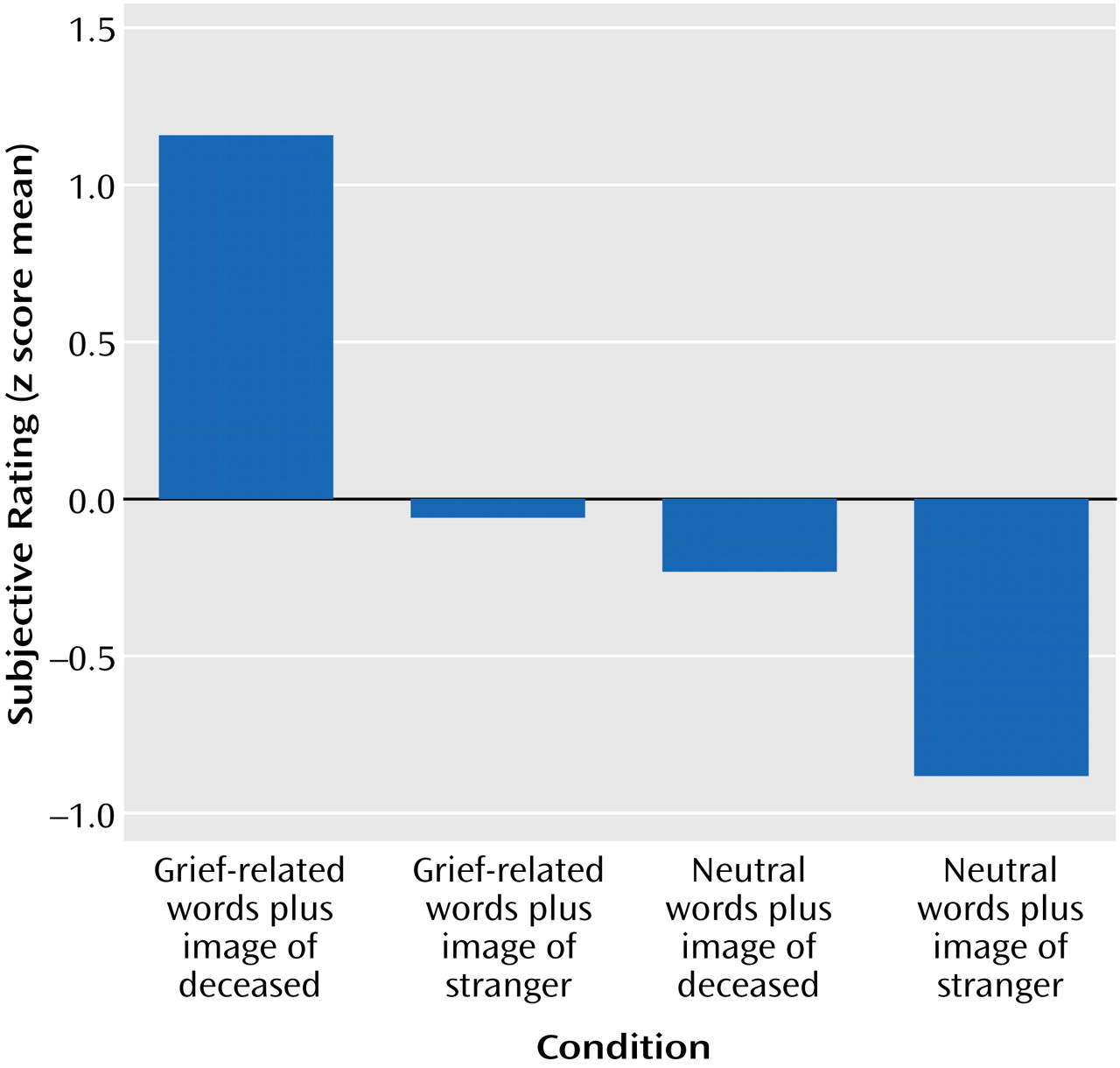
Across participants, the self-reported grief responses (on a 1–10 scale) to the picture-word composites were also significantly different for the four conditions. The mean scores were as follows: grief-related words plus photograph of deceased, 7.67 (range=4.87–9.93); grief-related words plus photograph of stranger, 4.23 (range=1.13–7.93); neutral words plus photograph of deceased, 3.52 (range=1.20–7.43); neutral words plus photograph of stranger, 1.75 (range=1.00–4.77). The z scores for the subjective grief responses were significantly different (
Figure 2), indicating that the grief-related stimuli elicited stronger responses. The correlation of the skin conductance responses with the self-reported responses was significant (r=0.25, N=480, p<0.01).
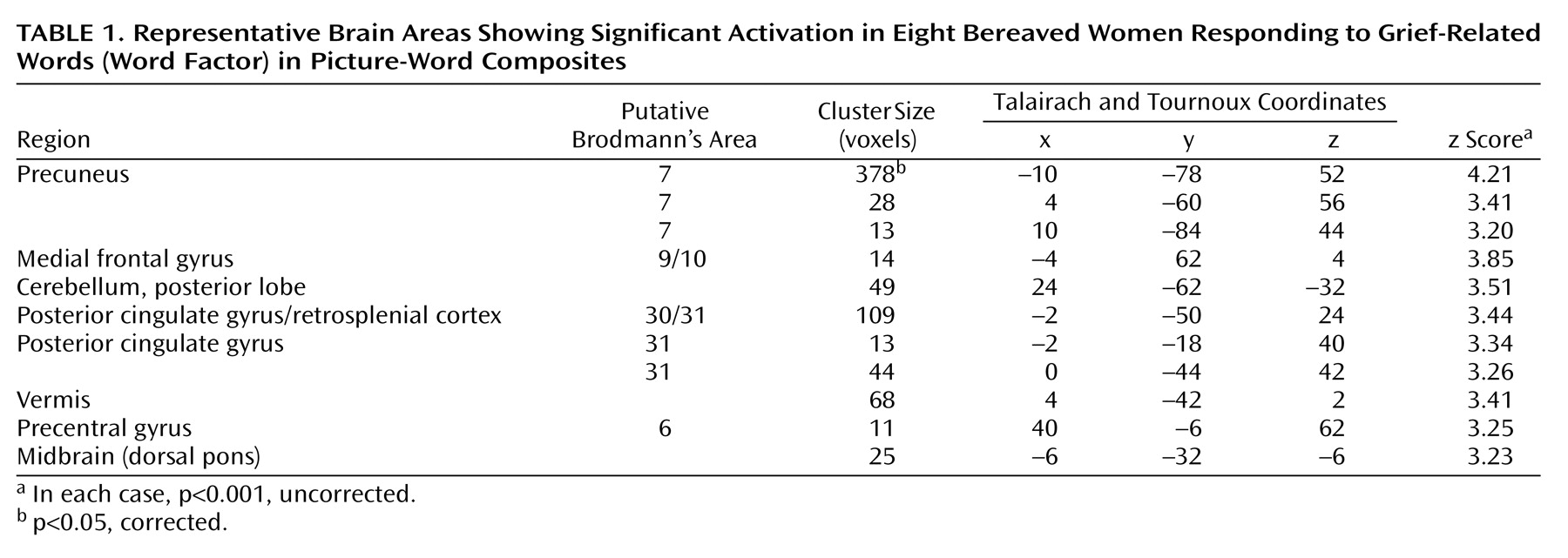
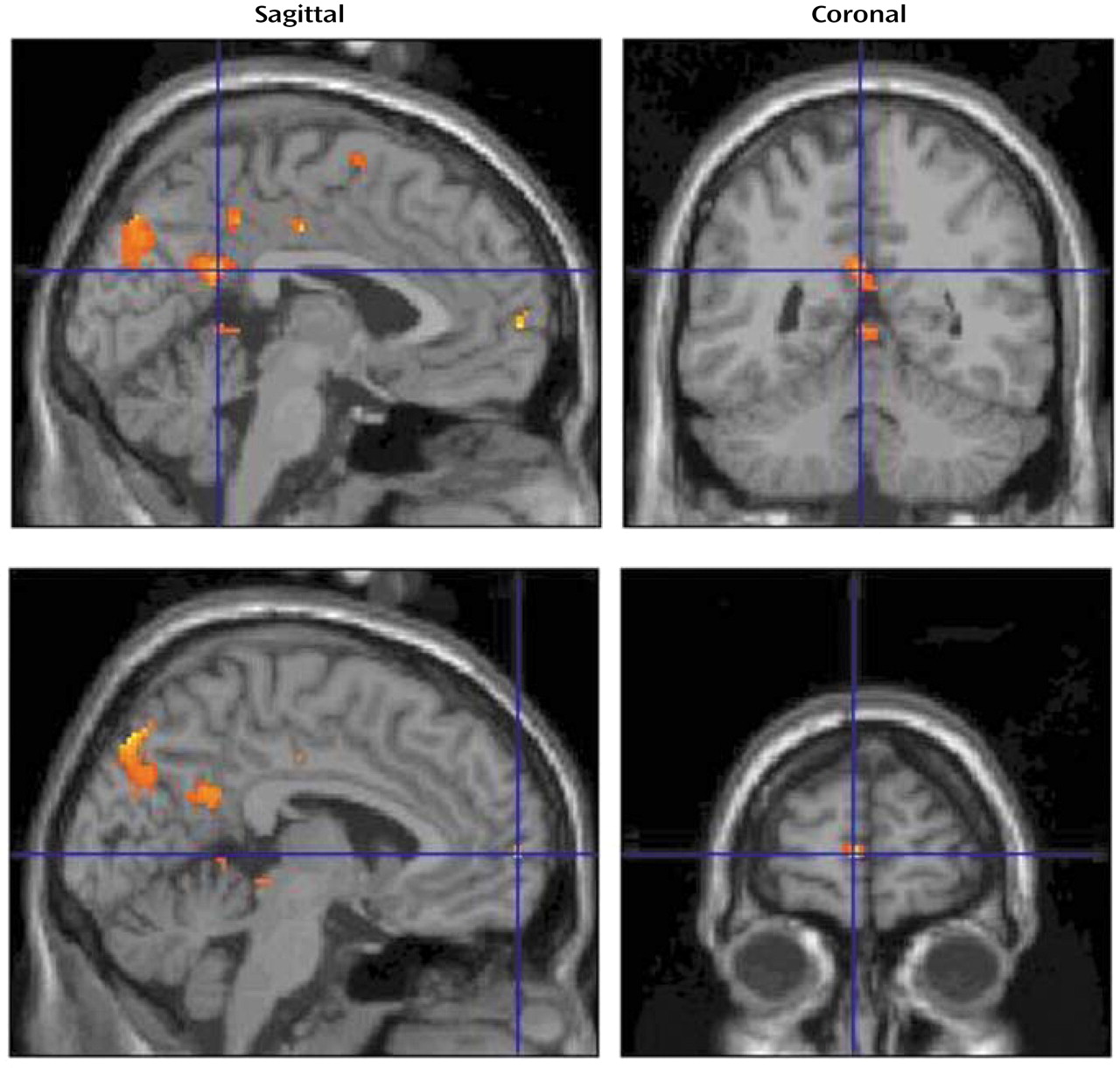
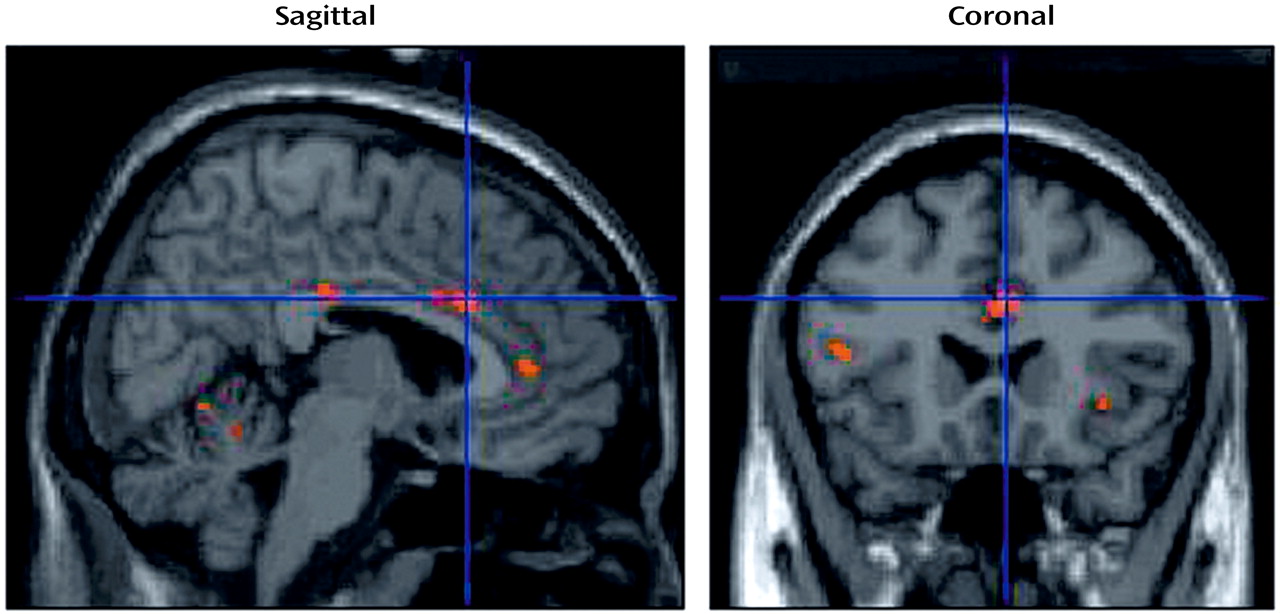
The brain areas activated during the verbal evocation of grief (word factor) are listed in
Table 1, and representative areas are displayed in
Figure 3. The table demonstrates that verbally induced grief was associated predominantly with activity of the bilateral precuneus, the left medial frontal gyrus, the bilateral posterior cingulate gyrus, the right precentral gyrus, the midbrain (dorsal pons), and various cerebellar subregions.
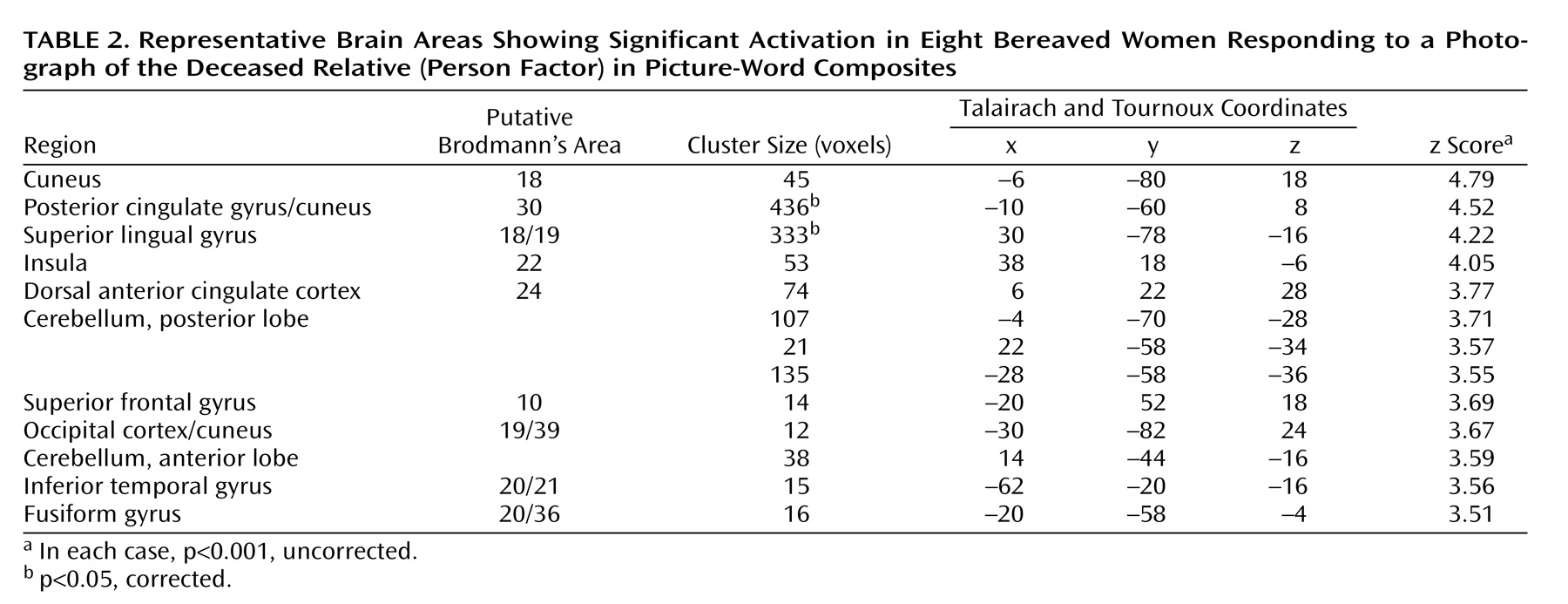
Table 2 and
Figure 4 show sites that were activated during the pictorial evocation of grief (person factor). Significant increases in neural activation centered on the left cuneus, the left posterior cingulate cortex/cuneus, the right superior lingual gyrus, the right insula, the right anterior cingulate cortex, the left superior frontal gyrus, the left inferior temporal gyrus, the left fusiform gyrus, and various cerebellar regions.
In the analysis of the interaction of the word factor and person factor, none of the predicted regions of interest (posterior cingulate cortex, anterior cingulate cortex, prefrontal cortex) showed significant activation. However, the cerebellar vermis (coordinates=6, –36, 2) showed significant activation (z score=3.69, cluster size=267; p<0.001, corrected).
In the single-subject analysis, the majority of the eight subjects showed a pattern of neural activation consistent with the predicted group results for verbal and pictorial evocation of grief. Five of the eight subjects showed increased neural activity in the posterior cingulate cortex/retrosplenial cortex (p≤0.05, uncorrected) associated with the word factor. In the cerebellum, seven subjects showed increased neural activity in the posterior lobe that was related to the person factor (p≤0.05, uncorrected). Seven subjects showed significant activation in the medial frontal gyrus associated with the person factor (p≤0.05, uncorrected). Five subjects revealed significant activation within the superior frontal gyrus in relation to the person factor (p≤0.05, uncorrected).
Discussion
In contrast to basic emotions such as sadness or fear, which may be elicited by using the same standard stimuli for all subjects, grief is a highly personal experience that is potentially difficult to elicit in the artificial neuroimaging environment. The picture-word composites used in this study to evoke grief were highly effective, as revealed by self-report and skin conductance data. Although the grief responses were genuine and intense, their duration was relatively brief, as indicated by the significant differences in skin conductance responses across the different conditions in this event-related paradigm. The combination of a general context (picture of the deceased or stranger) and a more specific memory elicitor (grief-related word or neutral word) was adapted from the procedure developed by Teasdale and colleagues
(16), in which emotional responses were successfully elicited by congruent but not by incongruent picture-caption pairs.
Our findings revealed that while the combination of the picture of the deceased and the grief-related words evoked the most intense grief response, the picture of the deceased elicited a moderate grief response when paired with the neutral word. Similarly, the grief-related word elicited a moderate grief response even when paired with the picture of the stranger. As such, the person factor and the word factor provide different routes into the neurobiological state of grief. These two perspectives provide both similar and dissimilar information about the neural substrates of grief, analogous to a radiologic examination of a given anatomical structure from more than one viewing angle. Consequently, we discuss the findings for the word and person factors as complementary and overlapping. Collectively these factors indicate that grief is mediated by a distributed network of neural structures that subserve affect processing, mentalizing, retrieval of emotion-laden episodic memories, processing of familiar faces, visual imagery, automatic motor responses, autonomic regulation, and modulation/coordination of this combination of functions.
The caudal posterior cingulate/retrosplenial region was activated for both the person and word factors. This complex receives major hippocampal projections
(17), has strong reciprocal connections with the parahippocampal and entorhinal cortices, and is intensely linked by reciprocal pathways to the dorsolateral prefrontal and anterior cingulate cortices. Thus, it may serve to connect the dorsolateral prefrontal cortex with the hippocampal formation. Activation of the caudal part of the posterior cingulate cortex is most strongly associated with the retrieval of autobiographical memories
(5). Lesions of the left posterior cingulate cortex have also been associated with the loss of verbal episodic memory and retrograde amnesia for personal events. Indeed, low activity in this region is the most prominent functional imaging finding in the earliest stages of Alzheimer’s disease
(18). In addition, the caudal posterior cingulate cortex is reciprocally connected to regions engaged in emotional processing, such as the anterior cingulate and orbitofrontal cortices. The posterior cingulate is consistently activated by emotionally salient stimuli and has been hypothesized to have a role in the interaction between emotion and memory
(5). Maddock
(19), in his review of the retrosplenial cortex, reported evidence that this structure participates in the encoding of the emotional significance of episodic memories, but he could not exclude its role in retrieval. The current study predominantly involved the latter and therefore supports its role in retrieval of emotion-laden episodic memories.
The precuneus was the area most robustly activated by the word factor. This region is part of the medial parietal cortex and is located superior and posterior to the retrosplenial cortex. It has widespread anatomical connections to the prefrontal cortex, the temporal and occipital cortices, and the thalamus
(20). The precuneus is a principal area involved in the conscious recall of memory-related imagery
(20) and may participate in the representation of polymodal imagery associated with successful memory retrieval
(5). The activation of this area by the word factor, as opposed to the person factor, could be due to specific memories and visual images associated with actual events elicited by the grief-related words.
The person factor, but not the word factor, was associated with activation in a variety of extrastriate visual processing areas, including the superior lingual gyrus, the cuneus, and the fusiform gyrus. Activation of the inferior temporal gyrus also suggests enhanced activity in object-recognition areas in the context of grief, particularly areas involved in the recognition and processing of familiar faces. Emotional arousal induced by visual stimuli can itself modulate activity in visual processing areas
(21,
22).
The person factor was also characterized by activity in the dorsal anterior cingulate cortex and the insula. The former is thought to play a superordinate role in executive control of attention
(23). Activation of the dorsal anterior cingulate is consistent with our hypothesis that emotional arousal associated with grief engages attentional resources. Another paralimbic area, the anterior insula, is also well established as an area specialized for the processing of visceromotor information
(24). Our procedure for eliciting grief was associated with significant skin conductance responses, supporting the interpretation that emotional arousal contributed to activation of these paralimbic regions.
Our finding of a significant activation in the left medial frontal gyrus (coordinates=–4, 62, 4) for the word factor corresponds to the regions associated with mentalizing, i.e., representing one’s own and others’ mental states
(25,
26). This suggests that the participants reflected on their own state of mind, as well as that of the person in the picture, during the elicitation of grief.
Bowlby
(27) viewed grief as a natural expression of what he called the “attachment behavioral system,” evoked to discourage prolonged separation of an individual from a primary attachment figure. Activation of the caudate nucleus was found during a functional neuroimaging study of romantic love
(28). In the current study we also observed activation of the caudate nucleus (coordinates=6, 6, 8) that was marginally below the cutoff value of z=3.09 (p<0.001). Caudate activation may reflect activation of automatic motor programs, associated with the feeling of “being drawn toward” the person depicted. If so, the caudate nucleus may contribute to the attachment system hypothesized by Bowlby.
Growing evidence suggests that the cerebellum contributes to affective, autonomic, and cognitive functions as well as to motor control
(8,
29). The cerebellar contribution to cognition is modulatory rather than generative. Damage to the cerebellar vermis leads to altered affectivity
(30,
31). Our findings show that not only the cerebellar vermis, but also the posterior and anterior lobes of the cerebellum, may indeed play an essential role in the coordination of emotional and cognitive functioning in the context of grief, as they were activated in response to both the person and word factors.
A limitation of the study is the absence of a comparison group. Without a comparison group of nonbereaved women, it is possible that grief was confounded with familiarity or attachment. However, two fMRI studies have investigated brain activation in subjects while they were remembering familiar people
(5) or viewing familiar faces and listening to familiar voices
(6). Both demonstrated activation predominantly of the posterior cingulate/retrosplenial cortex, but in contrast to our results, no anterior cingulate or cerebellar activation was observed. The dorsal anterior cingulate cortex is greatly, but not exclusively, influenced by emotional processing and may correlate with phenomenal awareness of emotion
(32), and the cerebellum may be the site where the intensity of emotional response is modulated
(33). The activation of these two sites within our study may represent a fundamental functional correlate of the intense feeling of grief beyond mere familiarity with the person.
Because of the subjective nature of grief, which precludes studies in animals, and the lack of previous neuroimaging work addressing the neural correlates of grief, our exploratory hypotheses included multiple areas of the brain. The relatively small number of study participants (N=8) and the heterogeneity of their relationships to the deceased loved ones are also potentially confounding factors. The present study should therefore be considered preliminary and should be followed by a larger, more definitive study with appropriate controls.
Identifying the neural correlates of grief may contribute to the elucidation of the physiological effects of bereavement on physical health
(34). For example, the “broken heart” phenomenon, the increased rate of sudden cardiac death within 6 months of conjugal bereavement, suggests that cardiovascular health is altered in bereavement
(35,
36). Additional research is needed to determine whether grief and depression
(36) exert adverse effects on cardiovascular health through similar mechanisms. Others have shown that traumatic grief symptoms predict a physical health event (e.g., cancer) months later
(2). Psychiatry stands in a unique position to explore these effects of grief on mental and physical health and to improve the lives of those touched by this powerful human experience.