Previous studies have reported high rates of smoking among the offspring of women who smoke during pregnancy. In two separate cohorts
(1), maternal smoking during pregnancy was associated with an elevated risk of lifetime smoking among adolescent female offspring but not among male offspring; this association was independent of mothers’ postnatal smoking and the child’s age. In two other studies
(2,
3), maternal smoking during pregnancy was an independent predictor of smoking among both male and female adolescent offspring. These studies suggest a positive association between maternal smoking during pregnancy and risk of smoking among offspring that is independent of the mother’s postnatal smoking.
A physiological link between maternal smoking during pregnancy and smoking among offspring is plausible because nicotine and other substances in cigarette smoke cross the placental barrier and may have direct and long-term effects on the neurological development of the fetus
(4–
6). The nicotine that passes from mother to fetus stimulates nicotinic receptors, which are present from the early stages of fetal development
(7). This activity may cause permanent abnormalities in the brain’s dopaminergic regulation
(8,
9). These effects, which may occur even at low nicotine doses and in the absence of notable fetal abnormalities
(10), may result in a greater liability to nicotine dependence than in those who have not been exposed to tobacco smoke in utero
(11). However, to our knowledge, there have been no prospective studies to date examining full nicotine dependence as a consequence of maternal smoking during pregnancy, although previous investigations have examined early stages of tobacco use. Accordingly, the aim of the present investigation was to determine whether maternal smoking during pregnancy resulted in an increased risk of nicotine dependence among adult offspring.
If maternal smoking during pregnancy affects pathophysiological processes that may lead to a heightened predisposition to nicotine dependence, we theorized that this link would be reflected in distinct patterns of smoking and nicotine dependence among offspring exposed to maternal smoking during pregnancy. First, although the focus of previous investigations has been the risk of ever smoking among the offspring of women who smoked during pregnancy, we expected that individuals exposed to tobacco smoke in utero would be at particularly greater risk of progressing from any cigarette use to regular use and dependence. Second, we expected that such individuals would be at especially elevated risk for nicotine dependence in contrast to dependence on other substances, such as marijuana. Marijuana use was of particular interest because of the similar route of administration (inhalation) and because of the known co-occurrence of smoking tobacco and marijuana use
(12). Accordingly, in this investigation we test the hypotheses that 1) maternal smoking during pregnancy is associated with a heightened risk of nicotine dependence and risk of progressing from tobacco use to tobacco dependence but not with a heightened risk of the early stages of smoking behavior and 2) maternal smoking during pregnancy predicts nicotine dependence but not marijuana dependence.
To our knowledge, this study is unique in three ways. First, it is the only study to use data on maternal smoking that have been collected prospectively and chemically validated. Second, it is the only study to date to examine the link between maternal smoking during pregnancy and nicotine dependence. Finally, it is the only study to examine this link over a span of 30 years among adult offspring.
Method
Providence Cohort of the National Collaborative Perinatal Project
Subjects were offspring of mothers enrolled in the Providence, R.I., site of the National Collaborative Perinatal Project (described in detail by Niswander and Gordon
[13]). Briefly, the National Collaborative Perinatal Project was a multisite cohort study that involved the prospective observation and examination of more than 50,000 pregnancies through the first 7 years of life. Data from mothers were collected beginning at the time of registration for prenatal care by trained staff using standardized protocols. Subject recruitment occurred between 1959 and 1966. Enrollment at each site was based on sampling frames designed to yield a representative sample of women receiving prenatal care at each of the 12 sites
(14,
15).
In Providence, obstetric patients at the participating hospital were randomly selected for inclusion in the study. On average, one out of two patients was chosen; the exact sampling fraction of patients was varied in order to recruit a predetermined number of subjects during each month of enrollment. The Providence cohort included 3,089 pregnant women. At the conclusion of the study, a total of 4,140 pregnancies had been enrolled.
Parental occupational and educational levels for the Providence cohort are generally comparable to those of the local and national populations, as reflected in 1960 Census data, with a slight skew toward participants of lower socioeconomic status
(16). The mean level of educational attainment for white male heads of household for the Providence cohort (11.0 years of education) was 0.5 standard deviations lower than the U.S. national average in 1960 (12.0 years). Moreover, comparison of the annual income of the Providence cohort with all Providence residents indicates that all income levels are included in the study sample, with a slight overrepresentation of lower income levels.
Follow-Up of the Offspring of the Participants of the Providence Cohort
Of the 4,140 Providence National Collaborative Perinatal Project pregnancies, 1,780 were selected for follow-up. Selection occurred in two separate phases; in each, a stratified random sample was drawn from the entire cohort to investigate the association between early life factors and adult substance use and psychiatric disorders. During the first phase, initiated in 1984, 995 eligible subjects with and without pregnancy/delivery complications were selected for follow-up and 692 interviewed
(16). In the second phase, initiated in 1996, 1,057 subjects with and without potential learning disabilities were selected and 720 interviewed
(17).
Interviews were typically conducted in person by well-trained lay interviewers; however, for approximately 15% of the sample, respondents could not be met in person and were interviewed by telephone by the same pool of interviewers
(16). Subjects were relocated by using a variety of methods, including contact with family members, use of phone and address directories, and Internet services. Following mailed introductions, researchers contacted the participants, described the study protocol, and scheduled interviews at the subject’s home or study offices. Subjects were compensated at the rate of $10 per hour for their time.
Adult follow-up interviews were conducted when the subjects were between the ages of 17 and 39 years; those individuals who were sampled during the first phase of the study were 17 to 27 years old, and those in the second phase were between 30 and 39 years old. Some subjects (N=146) were interviewed in both phases; however, information from only one interview per subject was used in the present investigation, yielding 1,266 individuals interviewed.
For participants in both phases, the second interview was used to permit a longer period of follow-up and thus more time for nicotine dependence to develop. Eighteen respondents were excluded because of missing data, yielding a final sample size of 1,248.
Study Variables
At the first prenatal visit, women reported whether they were currently smoking and, if so, the number of cigarettes they smoked per day. These questions were repeated at each subsequent prenatal visit up until the time of delivery. From these repeated measurements, we determined the maximum number of cigarettes smoked at any point during pregnancy. For the purposes of statistical modeling, women were classified into three levels of maternal smoking during pregnancy, according to the maximum number of cigarettes smoked on any pregnancy day: never smoked, smoked less than a pack during any pregnancy day, and smoked a pack or more during any of their pregnancy days. Information on the mother’s race/ethnicity and socioeconomic status, assessed during the first prenatal visit, was also collected. A composite index of socioeconomic status was calculated on the basis of methods developed by the U.S. Census Bureau. The index reflects education and occupation of the head of household along with household income
(18).
The nicotine and marijuana dependence of offspring were diagnosed according to DSM-III criteria. In the first phase, DSM-III diagnoses were generated by using the National Institute of Mental Health Diagnostic Interview Schedule (DIS), version III
(19); in the second phase the revised version III of the DIS
(20) was administered. Information on cigarette and marijuana use was comparable in the two versions; however, the item, “Have you ever smoked a cigarette?” was asked in the second phase of the study only.
Regular smokers were defined as those who smoked daily for 30 days or more at some point during their lifetime. Regular marijuana use was defined as daily use for 2 weeks or longer, consistent with the criteria used in the DIS. Human subjects approval was granted by human studies review groups at Harvard University and Brown University. Written informed consent was obtained from all interviewed study participants.
Data Analysis
We estimated relative risks with logistic regression modeling, using the SAS proc logistic version 6.12 (SAS Institute, Cary, N.C.). All models included two binary variables, one representing maternal smoking of less than a pack and the other representing maternal smoking of a pack or more, and offspring of nonsmokers were used as the reference group. The models also included variables for maternal age at pregnancy, family socioeconomic status at time of pregnancy, maternal race/ethnicity, offspring gender, and offspring age at time of interview. We accounted for the stratified sampling of respondents selected for the study by including a set of indicator variables representing the initial study selection factors (i.e., history of pregnancy or delivery complications and potential learning disabilities at age 7) in all models. Odds ratios for ever use, regular use, and dependence were estimated for the entire sample.
Odds ratios for progression from one stage of tobacco or marijuana use to another stage of use were calculated by conditioning the analysis on the earlier stage of use. For example, to estimate the odds of progressing from ever smoking to regular smoking, regular smoking was modeled in a sample limited to those subjects who reported ever smoking. Likewise, to model progression from regular use to dependence, dependence was modeled in a sample limited to regular users. For the gender-specific analyses, these procedures were followed separately among samples of male and female offspring.
Results
As shown in
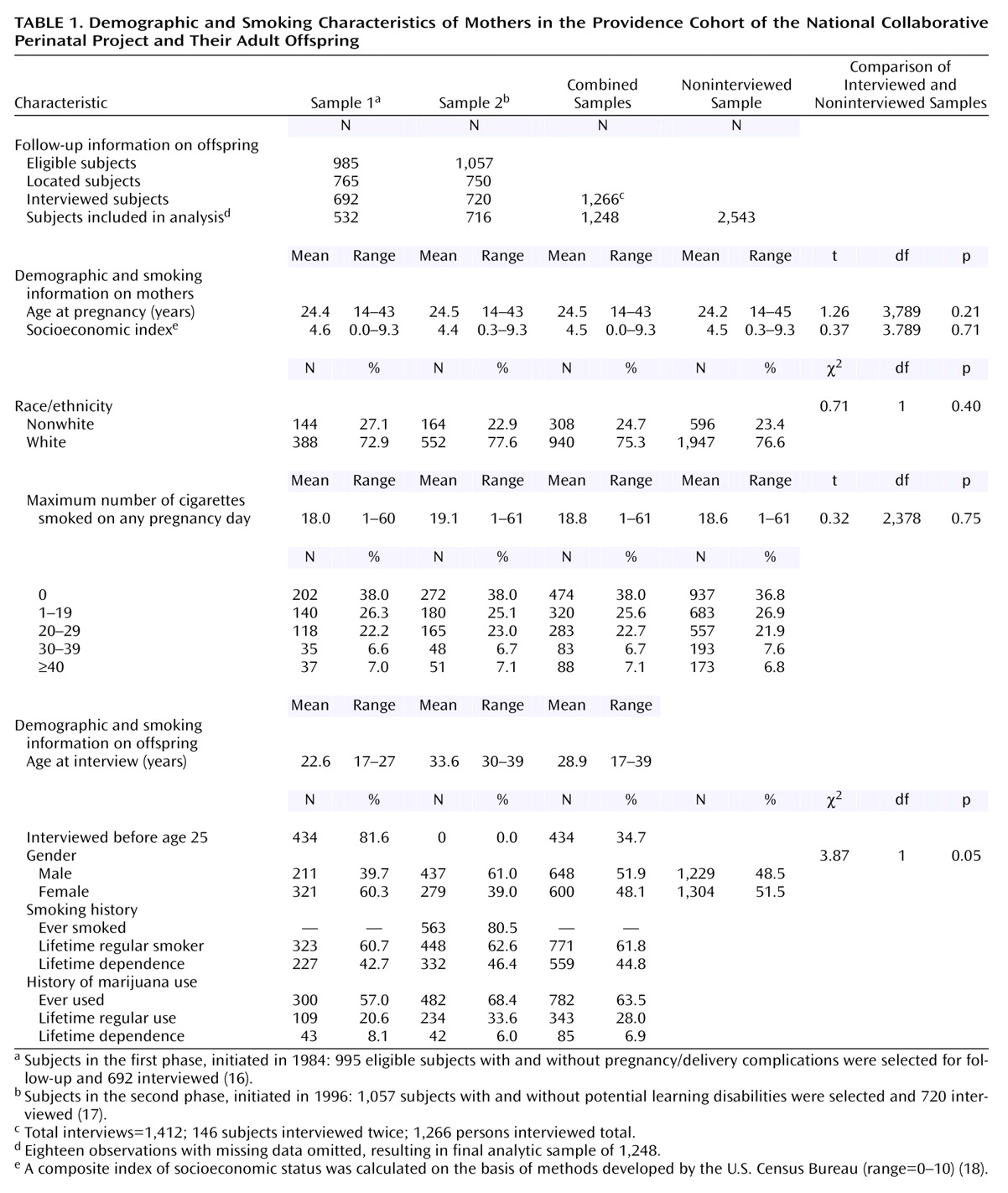
Table 1, mothers of offspring who were followed up for the two phases of the study did not differ significantly in terms of their age at pregnancy, socioeconomic status, race, or maximum number of cigarettes smoked on any day during pregnancy. By design, offspring in the two studies did differ according to the age at interview and gender. Since the second phase of the study was conducted approximately 10 years after the first phase, rates of smoking and marijuana use were generally higher for this sample. The two samples were combined for subsequent analyses.
Over half (62%) of the women smoked during their pregnancies. Among those women who smoked during pregnancy, the average number of cigarettes smoked during any pregnancy day was 18 cigarettes per day. Slightly over a quarter of the women reported smoking less than a pack, and about 35% smoked a pack or more. The women were on average 24.5 years of age at the birth of their offspring; there was no difference in the age at birth of mothers who were smokers or nonsmokers. The women had a mean family socioeconomic status of 4.5 (range=0–9.3) on a scale of 0–10. Smokers were of slightly but significantly lower socioeconomic status than nonsmokers (4.4 versus 4.6) (t=1.87, df=1,246, p=0.06); however, smoking during pregnancy was common among all socioeconomic status groups.
Slightly over half of the offspring were male (52%); the mean age of all offspring at interview was 29 years (range=17–39). By the time of follow-up, 62% had smoked regularly during their lifetime, and 45% met DSM-III criteria for lifetime nicotine dependence. Offspring were classified into three exposure categories according to the maximum number of cigarettes their mother smoked on any pregnancy day. Offspring of nonsmokers (N=474 [38.0%]) were the referent group. Two comparison groups were used in the analyses. The lighter exposure group consisted of offspring of mothers who smoked less than a pack during any pregnancy day (N=320 [25.6%]); the heavier exposure group consisted of offspring of mothers who smoked a pack or more during any of their pregnancy days (N=454 [36.4%]).
There were few differences between the analytic sample and the remainder of the study cohort. The interviewed subjects were comparable to the noninterviewed subjects in terms of mother’s age at time of offspring birth, socioeconomic status, and maximum number of cigarettes smoked during any pregnancy day. There was a slight overrepresentation of male subjects among the interviewed sample (
Table 1).
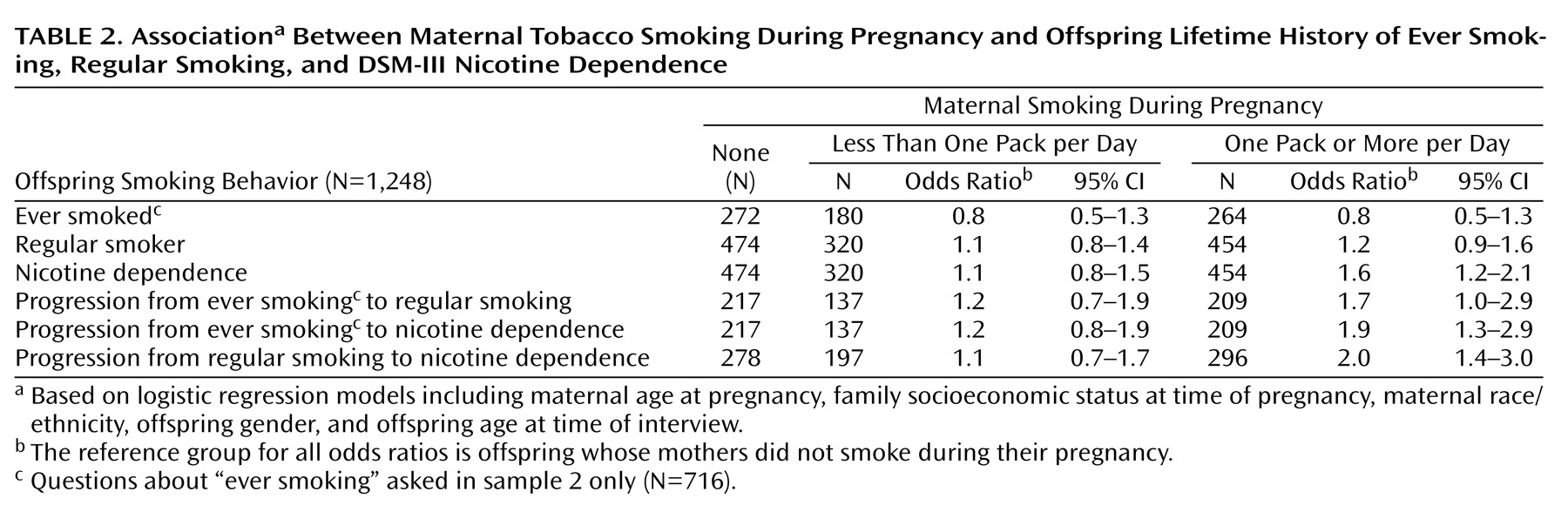
Results of logistic regression models predicting ever smoking, regular smoking, and nicotine dependence are presented in
Table 2. All models were adjusted for potential confounders, including mother’s age at pregnancy and race/ethnicity, family socioeconomic status, offspring’s gender and age at the time of interview, and sample selection variables. None of these smoking outcomes occurred more frequently among offspring whose mothers smoked less than a pack per day during pregnancy than in offspring of mothers who did not smoke during pregnancy (referent group).
Offspring of mothers who reported smoking one pack or more per day at some point during pregnancy had similar odds of ever smoking and becoming a regular smoker (smoking daily for 30 days or more) as offspring of nonsmokers. However, offspring of heavier smokers were more likely to become nicotine dependent than offspring of nonsmokers (
Table 2). This finding was most evident in the examination of the odds of progressing from ever smoking or regular smoking to nicotine dependence. Offspring of heavier smokers who ever tried cigarettes or who came to smoke cigarettes on a regular basis were approximately twice as likely to progress into full nicotine dependence (
Table 2).
Gender Differences
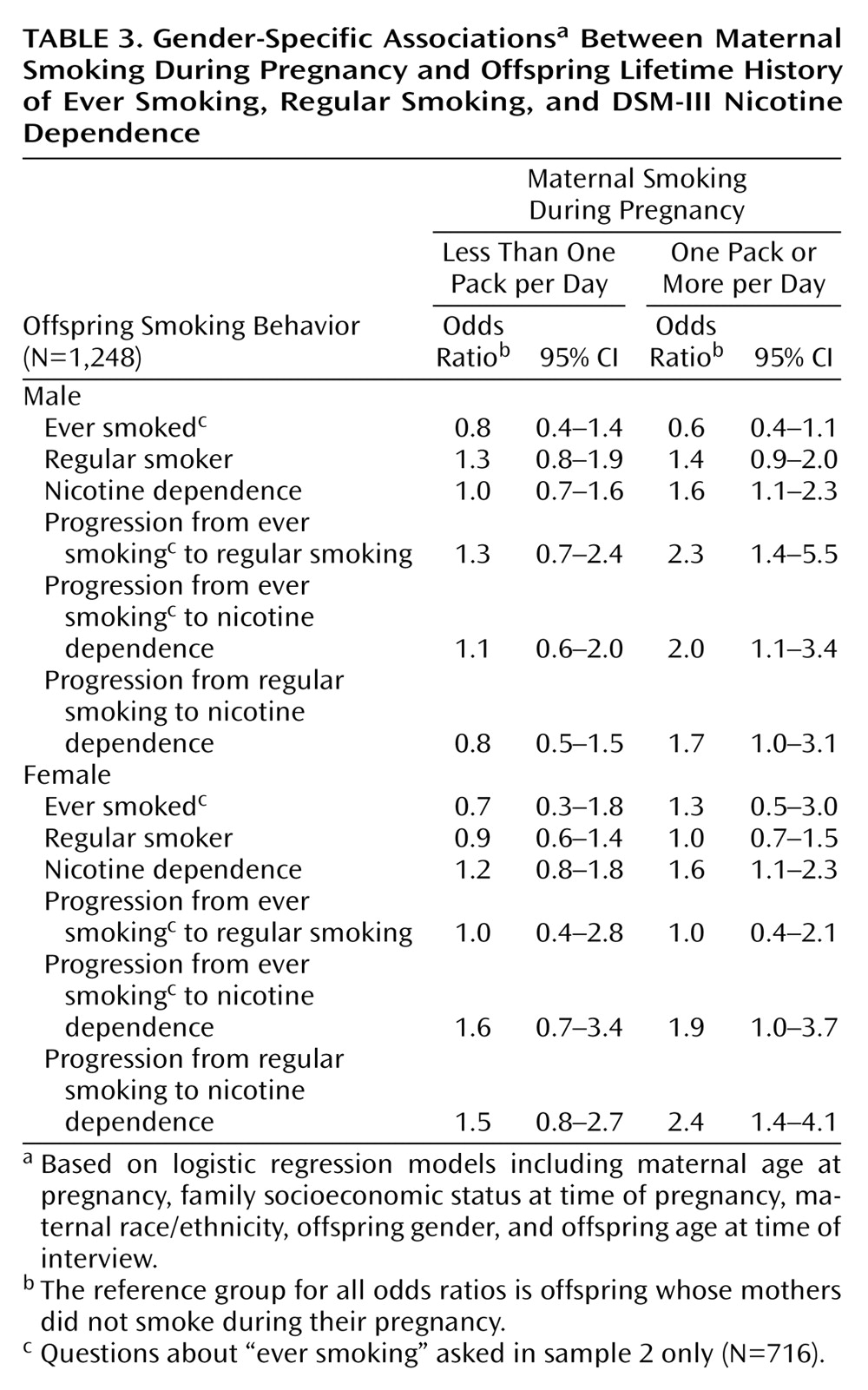
These models were recalculated separately for male and female offspring to examine possible gender differences in the association between maternal smoking during pregnancy and lifetime smoking patterns. As shown in
Table 3, there was no effect associated with moderate levels of maternal smoking during pregnancy (less than one pack per day) for either men or women.
For men, higher levels of maternal smoking during pregnancy were significantly associated with higher levels of all smoking outcomes other than ever and regular smoking. Male offspring of mothers who smoked a pack or more per day during pregnancy were approximately twice as likely to become a regular smoker, to progress from ever smoking to regular smoking, to become nicotine dependent, and to progress from ever smoking or regular smoking to nicotine dependence. The associations between maternal smoking during pregnancy and smoking were generally less pronounced for female offspring. Unlike the odds for male offspring, the odds of regular smoking or progressing from smoking to regular smoking were not elevated among female offspring of heavier smokers. For women as well as men, progression from regular smoking to nicotine dependence was statistically elevated among offspring of heavier smokers (
Table 3).
Specificity of Findings
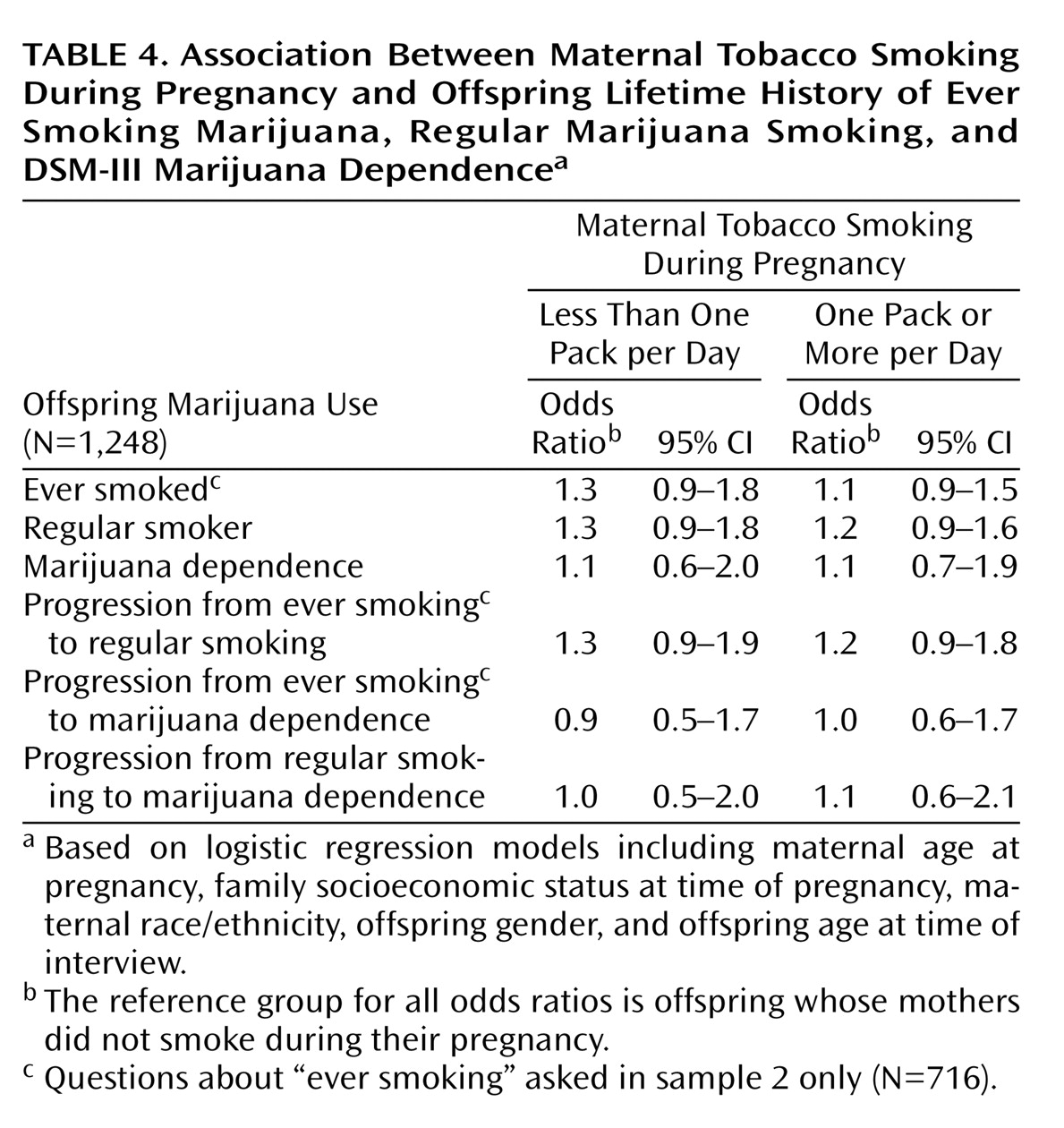
We conducted parallel analyses examining the association between maternal smoking during pregnancy and risk of lifetime marijuana use. As with cigarettes, we examined three levels of lifetime marijuana use: ever use, regular use (daily for 2 weeks or more), and marijuana dependence according to DSM-III criteria. Results of these analyses are presented in
Table 4. There was no association between maternal cigarette smoking during pregnancy and offspring marijuana use. In addition, gender-specific models (not shown) also indicated no association between maternal smoking during pregnancy and marijuana use among offspring.
All analyses were repeated separately for each of the two subsamples that were combined for the results already presented. The effect sizes were strikingly consistent in both subsamples, although certain estimates were no longer statistically significant because of reduced sample sizes.
Discussion
Our results point to a link between maternal smoking during pregnancy and the risk of nicotine dependence among adult offspring. This link is independent of family socioeconomic status, maternal age at pregnancy, offspring gender, and offspring age at the time of interview. Moreover, the elevated risk is found only for offspring of mothers who smoked at moderately high levels during pregnancy, namely, a pack or more on 1 or more days during pregnancy.
The lifetime impact of maternal smoking during pregnancy appears to be specific to nicotine dependence; neither ever smoking nor any form of marijuana use was associated with maternal smoking during pregnancy. The specificity of the link between maternal smoking during pregnancy and offspring nicotine dependence is in line with other work suggesting a pathophysiological pathway between maternal smoking during pregnancy and nicotine dependence (e.g., Kandel and Udry
[21]). Such specificity also suggests that any fetal perturbances associated with maternal cigarette smoking during pregnancy do not affect adult marijuana use and that the pathophysiological pathways for marijuana use are different from those for nicotine dependence.
Nicotine readily reaches the fetal compartment throughout pregnancy. The nicotine that reaches the fetus can directly affect fetal neural development, primarily through its action on acetylcholine receptors, which are present very early in the fetal brain. At least two types of enzymes necessary for the synthesis of acetylcholine are present in the brain as early as 8 weeks of gestation, suggesting that nicotinic signaling can significantly affect the brain’s neural development
(22). These effects are also likely to be long-lasting. Unlike in mature organisms, where stimulation of a target cell elicits a short-term response, in the immature systems, receptor stimulation interacts with the genes that control cell differentiation, thereby permanently altering the cell’s responsiveness. Changes in gene expression may affect the processes of cell replication, differentiation, function, and death. Moreover, the extensive functional interactions of cholinergic and catecholaminergic systems suggest that exposure to nicotine in utero is likely to affect multiple neurotransmitter pathways and the programming of synaptic competence
(7).
There are other potential explanations for the observed association. Mothers who smoked heavily may have passed on a genetic predisposition for smoking or other conditions such as depression that are associated with smoking and nicotine addiction. The link between maternal smoking during pregnancy and nicotine dependence, therefore, may also involve a genetic component
(21). However, heavy smoking was relatively common among National Collaborative Perinatal Project mothers in the 1960s; 34% of the sample smoked at least a pack of cigarettes during 1 or more days during their pregnancies. Consequently, it is unlikely that the observed patterns are solely due to genetic influences. Genetic factors may explain interindividual differences in sensitivity to the effects of exposure to nicotine in utero. One possibility is that exposure to nicotine in utero interacts with genetic influences, both contributing to the propensity for nicotine dependence among offspring
(21).
Another alternative explanation is that heavy-smoking mothers may have continued to smoke after the pregnancy and exposed their children to secondhand smoke. Although postnatal exposure to secondhand smoke may affect the propensity of offspring to nicotine dependence, it is unclear that brain levels of nicotine engendered by such exposure during childhood would be sufficient to alter nicotinic or other receptor function, density, or structure to predispose toward dependence subsequent to smoking initiation. However, for offspring of mothers who smoke and breast-feed, nicotine levels in breast milk are up to three times higher than in the mothers’ blood and could constitute a significant source of environmental nicotine exposure
(23).
A potential shortcoming of studies of this nature concerns the accuracy of mothers’ reports of smoking during pregnancy. However, in the present study mothers’ self-reports were mostly collected before the 1964 Surgeon General’s report, when smoking was less socially undesirable than it is now. More importantly, an analysis of 448 National Collaborative Perinatal Project participants demonstrated a significant agreement (kappa=0.83) between serum cotinine concentration derived from maternal sera stored for 40 years and maternal reports of smoking during pregnancy. Treating a serum cotinine concentration of >10 ng/ml as an index of active smoking, 95% of women who denied smoking and 87% of women who stated that they smoked reported their status correctly
(24). Offspring reports of smoking cigarettes and marijuana may have been influenced by a desire to appear socially desirable. However, social desirability concerns would lead to underreporting of use, weakening the observed association.
In conclusion, to our knowledge this study is the first to link maternal smoking during pregnancy with nicotine dependence in offspring. The link between maternal smoking during pregnancy and nicotine dependence is likely to be mediated by physiological (and/or genetic) pathways. A link between maternal smoking during pregnancy and nicotine dependence has important public health implications. In the United States, nearly half of all women smokers continue to smoke through their pregnancies
(25); this amounts to about 12% of all women who give birth
(26) and more than half a million infants who are annually exposed to cigarette smoke prenatally. To the extent that smoking contributes directly to fetal toxicity resulting in neural changes that increase dependence vulnerability, and also to the extent that genetic influences interact with the intrauterine environment to influence dependence, reducing rates of nicotine dependence among offspring may be one of the many anticipated benefits of and a rationale for intensified clinical and public health efforts to reduce maternal smoking during pregnancy.