Schizophrenia is a pervasive neuropsychiatric disease of uncertain etiology. Epidemiological and immunohistochemical studies indicate that environmental factors such as infections with members of the family
Herpesviridae may contribute to some cases of schizophrenia, most likely in combination with genetic determinants of disease susceptibility
(1,
2). We investigated the effect of valacyclovir, a herpesvirus-inhibiting medication, on the symptoms of schizophrenia.
Method
Participants were recruited from the outpatient centers of the Sheppard Pratt Health System and other agencies in the Baltimore area. The inclusion criteria were a diagnosis of schizophrenia or schizoaffective disorder by DSM-IV criteria, age 18–65 years, current treatment with antipsychotic medication that conformed to the relevant Patient Outcomes Research Team recommendations
(3), a stable medication regimen over the previous 21 days, the presence of residual symptoms attaining a total score of least 50 on the Positive and Negative Syndrome Scale
(4), and the ability to provide informed consent. Persons were excluded from the study if they had mental retardation or an underlying nonpsychiatric medical disorder. Written informed consent was obtained from all study participants.
Before the initiation of therapy, blood samples were obtained for the measurement of antibodies to the following
Herpesviridae: herpes simplex virus type 1, herpes simplex virus type 2, cytomegalovirus, Epstein-Barr virus, humanherpes type 6, and varicella-zoster virus. The assays were performed by using commercially available enzyme immunoassays (Viro-Immun Diagnostica, Oberrusel, Germany; Advanced Biotechnologies, Columbia, Md.) and assay methods that have been previously described
(5). For each assay, the seropositivity of an individual was defined by comparing the reactivity generated by the sera in the immunoassay with the optical density generated by standard sera provided by the manufacturer. Samples that were reactive to cytomegalovirus by immunoassay were further tested with Western blotting by using previously described methods
(6).
After a 2-week lead-in period in which participants were administered inactive medication, treatment was initiated with valacyclovir, 1 g administered orally twice a day for a maximum of 16 weeks. Both the valacyclovir and the inactive medication were administered in addition to the participants’ standard psychiatric medications. Assessments were made with the Positive and Negative Syndrome Scale at the onset of valacyclovir therapy and 2, 4, 8, 12, and 16 weeks after the initiation of therapy. Improvement in each score was calculated as a percentage decrease in the score from baseline. Measurements were performed by trained raters who were masked as to the viral antibody status of the participants.
Statistical analysis was performed by repeated-measures analysis of variance (ANOVA) of scores on the Positive and Negative Syndrome Scale of all 65 participants, with serologic status regarding the herpesvirus as the independent between-group factor and week of therapy as the within-group factor. The normality of the distribution of the scores was documented by tests for kurtosis and skewness. Covariant analyses were also performed by adding potentially confounding demographic and clinical variables as additional between-group factors. A critical value of alpha of 0.008 (0.05/6) was used in light of the testing for antibodies to six viruses.
Results
A total of 65 participants consented to participate in the study, started valacyclovir therapy, and had at least 1 follow-up measurement. The participants had a mean age of 42.7 years (SD=10.6); a total of 47 (72.3%) were male, and 60 (92.3%) were Caucasian. The mean duration of schizophrenia was 22.4 years (SD=10.4). A total of 58 participants completed 16 weeks of therapy. One patient withdrew because of a possible side effect of valacyclovir (rash); the other six dropouts withdrew voluntarily for reasons apparently unrelated to the valacyclovir medication.
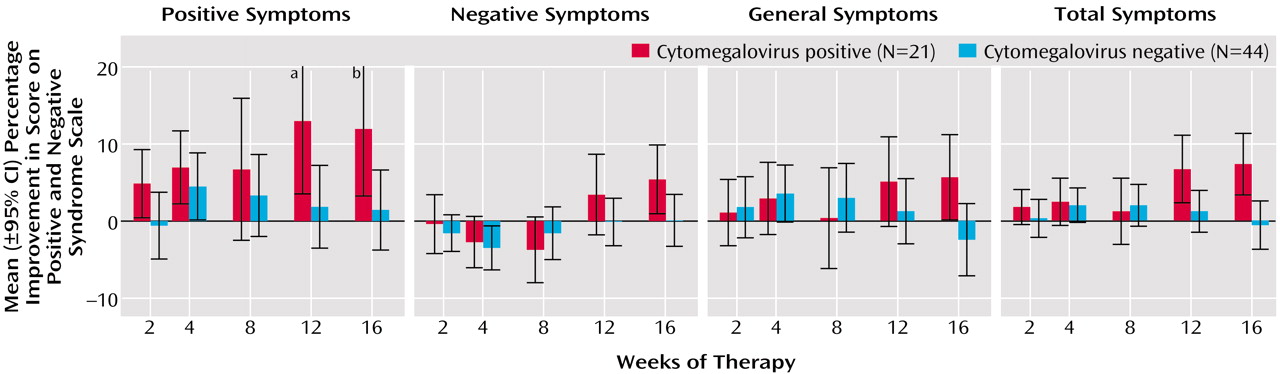
We did not find an association between improvement in scores on the Positive and Negative Syndrome Scale and seropositivity for herpes simplex virus type 1, herpes simplex virus type 2, Epstein-Barr virus, varicella-zoster virus, or human herpesvirus 6. However, as depicted in
Figure 1, individuals who were seropositive for cytomegalovirus displayed a significant improvement in overall symptoms, i.e., the Positive and Negative Syndrome Scale total symptom score (F=4.61, df=5, p<0.0005). The effect size of the improvement in overall symptoms in cytomegalovirus-seropositive individuals was 0.85. Some degree of improvement was noted in positive symptoms (F=2.48, df=5, p<0.04), negative symptoms (F=1.84, df=5, p<0.11), and general symptoms (F=2.46, df=5, p<0.04). Improvement was most evident after 8 weeks of valacyclovir therapy.
A total of 21 (32. 3%) of the participants were cytomegalovirus seropositive by enzyme immunoassay. The sera of these individuals also reacted to specific cytomegalovirus proteins on Western blot.
We also analyzed the effects of potential confounding variables on the interaction between cytomegalovirus seropositivity and improvement in the Positive and Negative Syndrome Scale total score. Cytomegalovirus seropositivity remained significantly associated with improvement when any of the following variables was added to the repeated-measures ANOVA model: age, duration of illness, number of hospitalizations, gender, race, marital status, initial Positive and Negative Syndrome Scale scores, diagnostic subtype, and type of antipsychotic medication.
Discussion
Valacyclovir, the orally administered l-valyl ester of acyclovir (acycloguanosine), is rapidly converted to acyclovir after ingestion. The administration of the prodrug rather than the native compound results in significantly higher levels of acyclovir in the blood and CNS
(7). Acyclovir is converted to its monophosphate derivative by kinases encoded by several different herpesviruses but not by enzymes present in uninfected human cells. The monophosphate is further phosphorylated to the corresponding triphosphate by cellular enzymes, resulting in the inhibition of viral DNA synthesis and viral replication by a number of mechanisms. Clinical trials have documented that valacyclovir is effective in preventing infections due to herpes simplex virus type 1, herpes simplex virus type 2, and cytomegalovirus in susceptible individuals
(8).
Our findings indicate that treatment with valacyclovir over 16 weeks can result in some degree of symptom improvement in individuals with schizophrenia who have serological evidence of cytomegalovirus infection. Valacyclovir may be more effective in this regard than native acyclovir, which has been previously reported to be ineffective in altering the symptoms of schizophrenia after oral administration
(9). The biological mechanisms of the clinical response to valacyclovir are not known with certainty but are likely to be related to its effect on the replication of cytomegalovirus. Because we did not obtain cerebrospinal or other body fluids for virologic analyses, we could not directly define the relationship between cytomegalovirus replication within the CNS and clinical response to valacyclovir. We also could not determine the effect of latent cytomegalovirus infection on patient symptoms. The definition of these relationships should be the subject of future investigations.
Our exploratory study should be replicated with other groups of individuals with schizophrenia by means of a placebo-control format as well as additional dosing regimens and lengths of treatment. Controlled studies should also be performed with additional medications that have anticytomegalovirus activity to further define the relationship between cytomegalovirus infection and the symptoms of schizophrenia.