Genes may not account for all of the susceptibility to schizophrenia, but they clearly play a considerable role in the etiology of the illness. Although the disorder is highly heritable, genetic linkage studies have failed to endorse schizophrenia linkage universally with any chromosomal region
(5). The difficulty finding genes for schizophrenia suggests that many genes combine to cause the disorder and that each confers only a small degree of risk
(6). Because evidence implicates dopamine system dysfunction in the pathogenesis of this disorder, the search for susceptibility genes has included those which code for all five known dopamine receptor subtypes
(7–
10), the dopamine transporter
(11), and several enzymes involved in the synthesis and degradation of dopamine, such as tyrosine hydroxylase, aromatic
l-amino acid decarboxylase, and monoamine oxidase (MAO) A and MAOB
(12–
14). None of these genes has reliably emerged as a risk factor for schizophrenia.
Because it codes for one of the major enzymes catalyzing the metabolism of dopamine, the gene for catechol
O-methyltransferase (COMT) (EC 2.1.1.6 according to the International Union of Biochemistry and Molecular Biology enzyme nomenclature) has been examined numerous times for an association with schizophrenia, but with equivocal findings. This ambiguity parallels that of genetic linkage analyses in the vicinity of the COMT gene on chromosome 22q11.21. Some, but not all, studies
(15,
16) have found evidence of linkage with schizophrenia in this region. However, a meta-analysis of genome-wide linkage scans
(17) identified chromosome 22q as one of three loci (along with chromosomes 8p and 13q) that had the highest likelihood of harboring schizophrenia-risk genes, which highlights the promise of COMT as a candidate gene for this disorder.
Because the conflicting results from association studies have obscured COMT’s true role in schizophrenia, we performed the meta-analysis reported on here to determine if the failure to identify an association consistently is attributable to the low power of individual studies to detect a small effect, etiological heterogeneity, or random error in the absence of a true effect.
Discussion
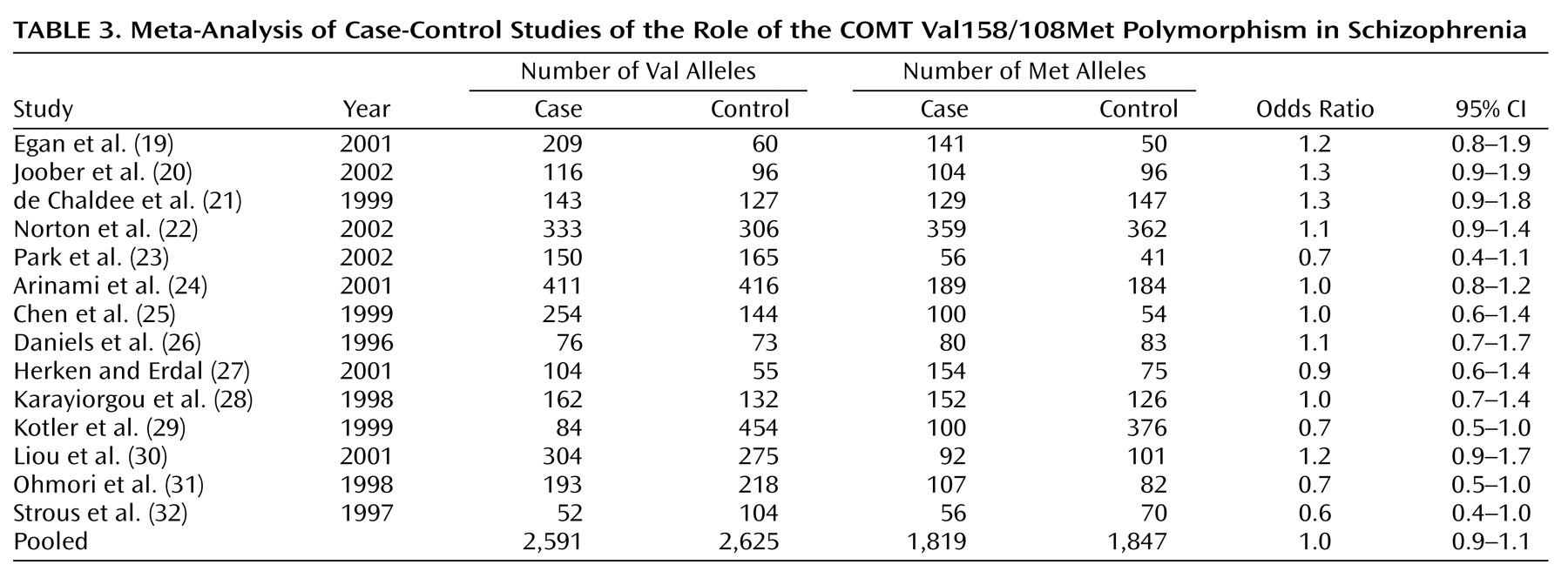
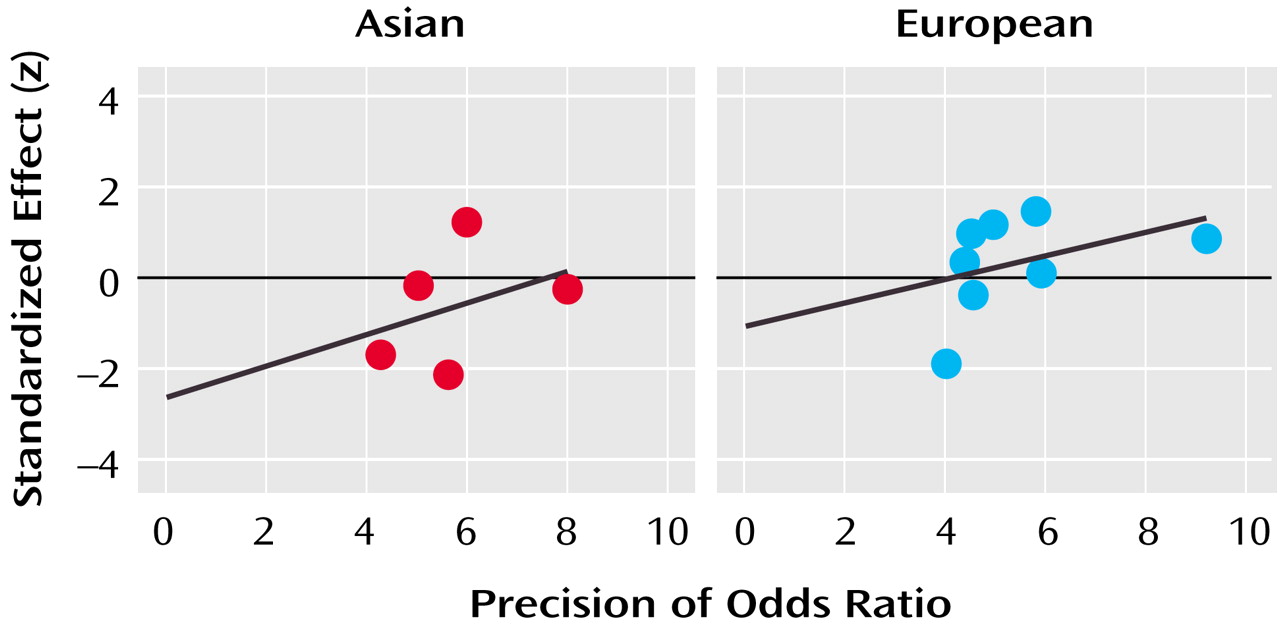
The pooled sample of 14 case-control studies had more than 90% power to detect a small but significant association (odds ratio=1.2) between COMT Val158/108Met alleles and schizophrenia but failed to identify such a relationship. However, rather than reflecting a lack of association, this may have been due to the statistically significant heterogeneity among studies. Overrepresentation of violent patients with schizophrenia in the sample did not appear to skew the results, as one such study
(29) found an odds ratio of 0.7 while the other
(30) found an odds ratio of 1.2.
The inclusion of schizophrenia spectrum conditions (e.g., schizoaffective disorder or schizotypal personality disorder) in a broader definition of the schizophrenia phenotype also failed to systematically affect the results, as one such study
(19) yielded an odds ratio of 1.2 while the other
(23) produced an odds ratio of 0.7. Other study characteristics, such as the gender composition of the sample or the age of the control group, did not account for this heterogeneity either. The only factor that reliably influenced the odds ratio obtained from each study was the ethnicity of the sample: the pooled odds ratio from Asian samples was 0.9, slightly lower than the pooled odds ratio of 1.1 from European samples.
In isolation from the findings of the five family-based association studies, several possible conclusions can be drawn from these observations. First, COMT may be a susceptibility gene for schizophrenia, but an overall association between the gene and the disorder may have been obscured by etiological heterogeneity whereby different COMT alleles confer risk to different ethnic groups. The present findings provide limited support for such an interpretation; however, it is important to note that neither allele was significantly associated with risk in either ethnic group. It is also possible that allelic variants of the COMT gene confer risk for schizophrenia to only a restricted segment of the population if at all, or that the COMT genotype is a universal risk factor of such small magnitude that its attributable fraction in the population is negligible.
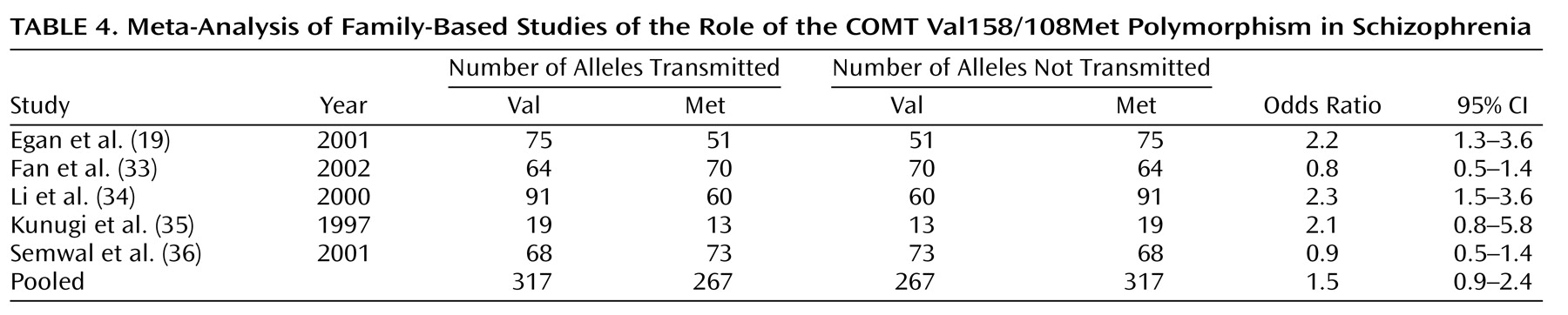
The five family-based studies help to clarify the nature of the relationship between the COMT Val158/108Met polymorphism and schizophrenia. Like the group of case-control studies, the family-based studies were not a homogeneous group, and this heterogeneity may have been attributable to a different pattern of genetic risk among Asian and European samples. The only two family-based studies of European samples provided nearly identical odds ratios that strongly implicated the Val allele in schizophrenia. This finding, in combination with the trend observed in the case-control studies, suggests that the Val allele may be a small but reliable risk factor for schizophrenia among those of European descent. In contrast, the findings from family-based studies of Asian samples were more variable as a whole and did not detect a significant association of schizophrenia with the Val allele. Thus, the family-based studies reinforced the possibility that the Val allele of this COMT polymorphism confers a different degree of risk to these two ethnic groups.
The variability of the evidence from Asian samples prevents firm conclusions about the role of this polymorphism in schizophrenia susceptibility in this population, and even though the Val allele was implicated more strongly in schizophrenia risk among European samples, this association seems tenuous at best. This relationship is further complicated by the possibility that the causative risk gene that produced this evidence for association is not COMT itself but a nearby gene that is in linkage disequilibrium with it. Clearly, more family-based studies of this putative association are sorely needed and will prove extremely informative. Determining the true nature of the relationship between this polymorphism of COMT and schizophrenia also may be facilitated by the application of emerging genomic-control association methods. The use of genotypes at randomly selected markers throughout the genome to control for population substructure in a case-control design provides a more powerful alternative to family-based association testing that nonetheless remains robust to false positive or false negative associations
(42).
There is some division in the conclusions to be drawn from case-control and family-based studies of the association between the COMT Val158/108Met polymorphism and schizophrenia. The former set of studies implicated the Val allele as a very weak risk factor in European samples (odds ratio=1.1), while the family-based studies place the effect of Val in European samples much higher (odds ratio=2.2). There is no simple resolution to this discrepancy, but the most conservative conclusion would be that, without additional evidence, we simply cannot determine if the COMT gene is implicated in schizophrenia. As new studies emerge, the present findings can be updated, and, eventually, more reliable estimates of this association in both ethnic groups may be obtained.
If, however, the present findings are already accurate estimates of the true effects to be obtained from each type of study and if the results from case-control and family-based studies do not converge upon the addition of more samples, then other possibilities must be considered. One alternative interpretation might be that case-control and family-based studies ascertain different populations of schizophrenic patients, and that this COMT polymorphism has a stronger contribution to risk in patients who become involved in family-based studies. The most obvious difference between the two groups of sampled patients is that those eligible for a family-based study must have two parents who are both willing and able to participate in the research. The availability of both parents could reflect a number of differences between patients enrolled in the two types of studies, such as a higher level of familial involvement with the patient and a better social support system or less severe pathology.
In the light of these discrepancies, there is reason to believe that the findings from the group of family-based studies (i.e., European samples, Val odds ratio=2.2) may be more accurate than the findings from the group of case-control studies (i.e., European samples, Val odds ratio=1.1). For example, the family-based study design is less susceptible than the case-control design to inferential errors based on artifacts like population stratification. As can be seen from
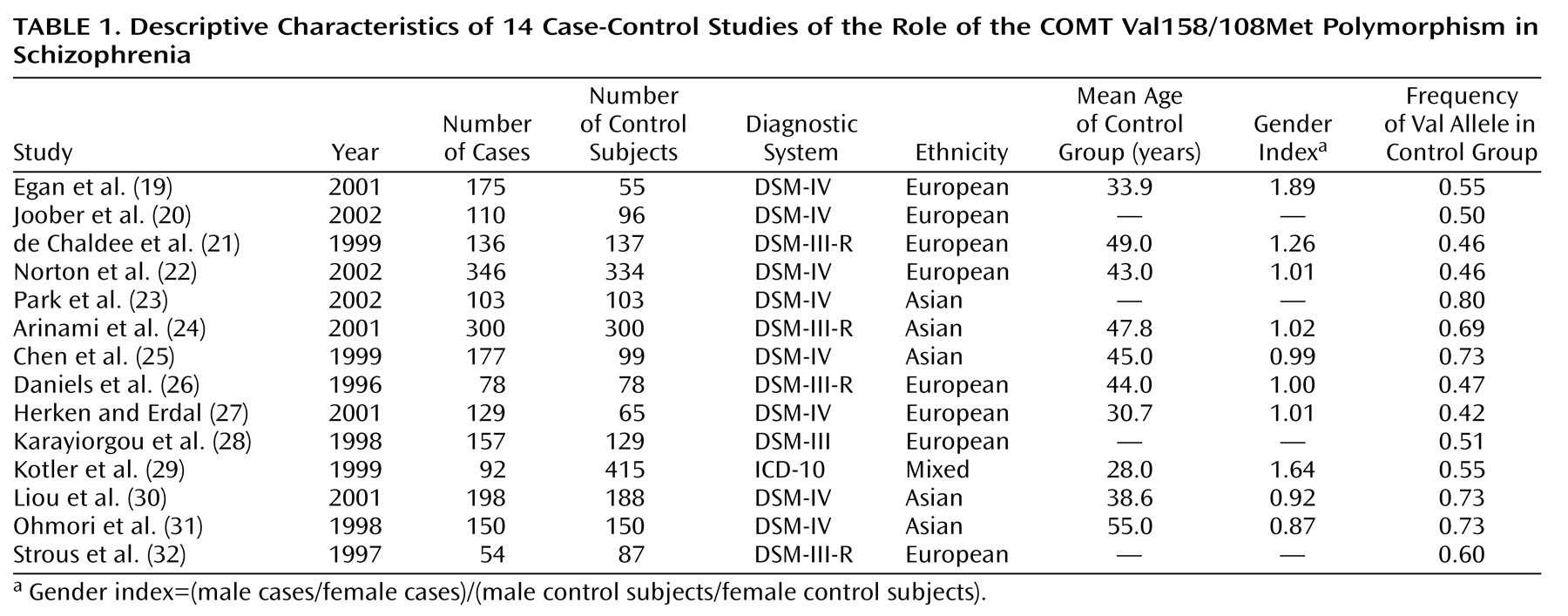
Table 1, the frequency of the Val allele varies widely across studies, and especially across studies of different ethnic groups. In fact, allele frequency in control groups was highly correlated with ethnicity in this group of studies (r=0.93, p=0.000005), and a significantly higher Val frequency was observed in Asian control samples (0.73) than in European control samples (0.49). These estimates are quite similar to allele frequencies previously reported for each of these populations
(43). Thus, despite the efforts to match cases and control subjects in population-based studies, even slight differences in ethnicity (and, consequently, allele frequency) between cases and control subjects can mask the contribution of a gene to the risk for a complex disease and invalidate estimates of association
(44).
In addition, the odds ratios derived from the two family-based studies of European samples were strikingly similar in their direction and magnitude, which further suggests they are detecting a significant, albeit small, association. Since a formal analysis of publication bias was not possible in the small group of family-based studies of European samples, it remains possible that evidence against the family-based association of Val with schizophrenia exists but is unpublished. Thus, it may be that a positively biased sample of the family-based studies was obtained and that the inclusion of only a few more negative studies would close the gap between the pooled outcome of case-control studies and the family-based studies of European samples. This possibility is reasonable given the well-known bias against the publication of negative findings in scientific journals
(45) and the reluctance of investigators to publish such findings.
The Val allele of this COMT polymorphism may increase susceptibility to schizophrenia, but it is important to recognize that this gene variant may also influence susceptibility to other psychiatric conditions. For example, this polymorphism is also associated with the rate of cycling in bipolar disorder
(46,
47). The Val allele may give rise to multiple physiological abnormalities that separately contribute to both of these illnesses, or it may produce a single deficit that is common to both disorders. It is not yet clear if the observed effects of the Val158/108Met genotype on executive cognitive functioning and prefrontal cortical activation during the performance of a working memory task
(19,
20) support the former or latter conclusion, since the specificity of these deficits for schizophrenia is unresolved
(48). However, COMT resides on chromosome 22q, which is one of only two regions (along with chromosome 13q) that showed significant genome-wide linkage for both schizophrenia and bipolar disorder in a recent meta-analysis
(17), further supporting a common effect of this polymorphism in both disorders. Although firm conclusions must await a large, definitive study, the present findings, along with the biological relevance of the Val allele, suggest that it may be considered a risk factor for schizophrenia, especially in those of European descent.