Brain imaging studies
(1–
6) have demonstrated volumetric white matter deficits (primarily in the frontal lobe and in the corpus callosum) among patients with schizophrenia. In addition, several studies have used diffusion tensor imaging to specifically characterize potential CNS white matter abnormalities in schizophrenia. (Although not absolutely correlated with morphological structure, the displacement distribution of water as measured by diffusion tensor imaging can provide information regarding microstructure, organization, and integrity of tissue
[7].) Four of six studies using diffusion tensor imaging
(8–
13) have indicated differences in diffusion measures suggestive of abnormalities in white matter structure in schizophrenia.
Data from structural magnetic resonance imaging (MRI) studies in schizophrenia have further indicated that white matter abnormalities may be specifically associated with negative symptoms
(2–
4); these data include our prior finding
(3) that patients with high levels of negative symptoms have the lowest white matter volume in inferior regions. Diffusion tensor imaging is a useful modality for probing white matter structures, and we know of no studies that have used this method to examine the potential relationship of frontal white matter microstructure and negative symptoms. Therefore, we conducted diffusion tensor imaging studies of 10 patients with schizophrenia who had varying degrees of negative symptoms. These studies were performed in order to test the hypothesis that negative symptoms are associated with abnormalities in inferior frontal white matter.
Method
After complete description of the study to the subjects, written informed consent was obtained from 10 male schizophrenic in- and outpatients. The patients were all under 50 years of age and met the DSM-IV criteria for schizophrenia. Patients with depression meeting diagnostic criteria, drug abuse/dependence within the last year, or history of neurological disease were excluded. The subjects were selected on the basis of a priori clinical familiarity in order to obtain a range of negative symptom severity. All subjects were receiving antipsychotic medication. The clinical rating measures included the Brief Psychiatric Rating Scale (BPRS)
(14) and the Schedule for Assessment of Negative Symptoms (SANS)
(15). Ratings were obtained when the patients were considered to be at maximal clinical stability (“baseline”), up to 40 days after the MRI scan. For the statistical analyses, negative symptom ratings were reduced to the SANS subscale global scores, a single total of the SANS global subscores, and the score on the withdrawal-retardation factor from the BPRS.
To minimize artifacts induced by eddy currents, the diffusion tensor imaging data were acquired by using a double-echo pulsed-gradient echo planar imaging method without cardiac gating, with TR=6 sec, TE=100 msec, field of view=24 cm, 128×128 matrix reconstructed to 256×256, b=1000 sec/mm
2, four averages, six noncollinear directions aligned to the anterior-posterior commissure (AC-PC) plane, and acquisition time=2 minutes, 36 seconds. Fractional anisotropy and mean diffusivity were computed as described in a previous report
(16). All raw and processed images were examined for artifacts by a reviewer who was blind to subject identity (K.O.L. or S.J.C.).
Standardized circular regions of interest were positioned, by an investigator blind to subject identity (S.J.C.), on the T2-weighted image (b=0) of the diffusion tensor imaging data set; thus, coregistration of images across different MRI acquisition methods was not needed. A single region of interest was placed bilaterally in the frontal white matter of five consecutive 5-mm-thick slices. These slices included three that were above the index slice, the index slice itself (defined as the most inferior slice containing the genu of the corpus callosum—approximately 10 mm above the AC-PC plane), and one slice below. For the three most superior frontal slices, large regions of interest (area=84.4 mm2) were centered in white matter with the posterior boundary of each region of interest on the imaginary line formed across the anterior most tips of the two frontal horns of the lateral ventricles. For the two most inferior frontal slices, medium-sized regions of interest (area=43.5 mm2) were centered in white matter with the posterior boundary of the region of interest on the imaginary line linking the most lateral extent of the sylvian fissures across the hemispheres.
Spearman’s rank order correlation coefficients were calculated to assess the relationship of negative symptom variables with white matter fractional anisotropy and mean diffusivity in the five frontal regions. To reduce the number of comparisons, we averaged fractional anisotropy and mean diffusivity across hemispheres to yield one fractional anisotropy and mean diffusivity value per slice. For these analyses, alpha was set at 0.01, two-tailed. The reported results represent the only hypothesis regarding anatomic regions and symptom scores that was tested.
Results
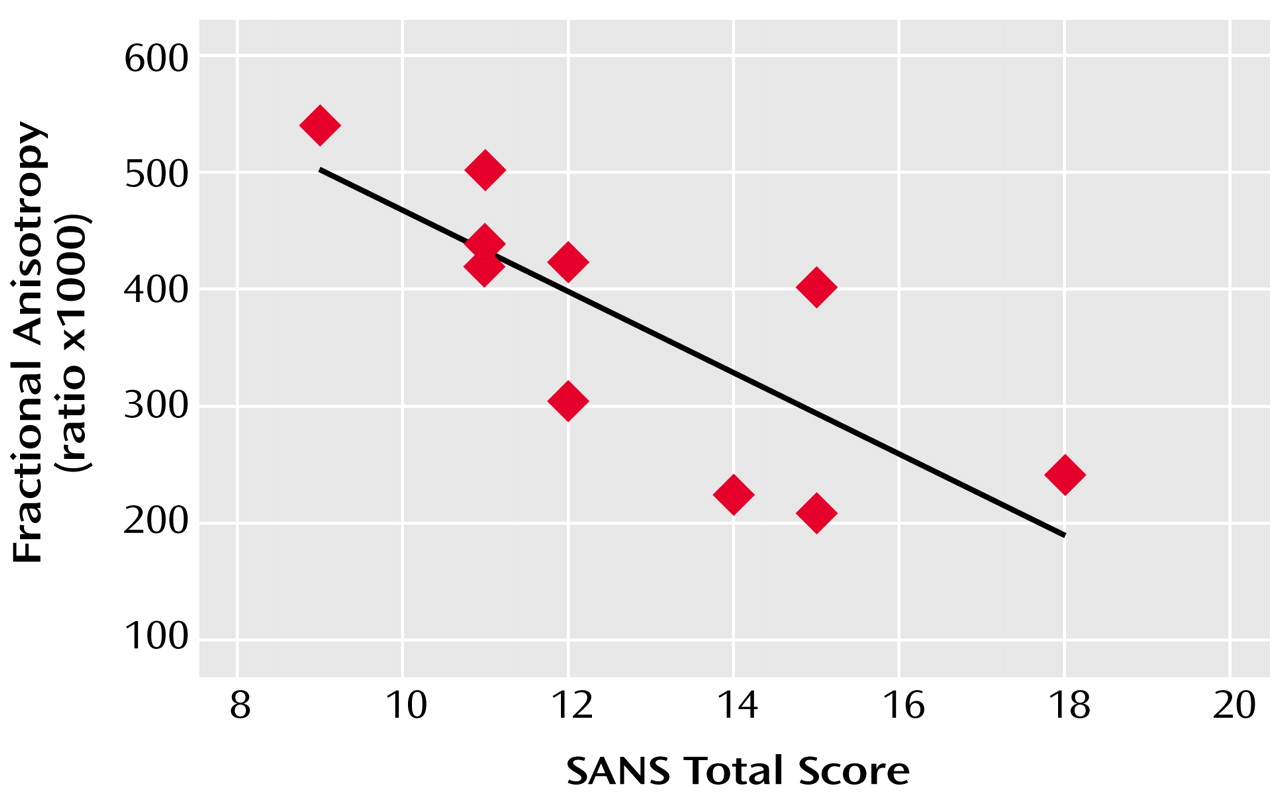
The mean age of the 10 patients was 41 years (SD=9); their mean duration of illness was 17 years (SD=9), and their mean sum of SANS global subscores was 12.8 (range=9–18).
Across subjects, frontal white matter fractional anisotropy at 5 mm below the AC-PC plane was inversely correlated with the severity of affective blunting (r=–0.72, p=0.02) and anhedonia (r=–0.69, p=0.03), according to the SANS subscales, and with a higher overall sum of SANS global subscores (r=–0.84, p=0.002) (
Figure 1). Similarly, greater withdrawal-retardation, indicated by the BPRS, was inversely correlated with white matter fractional anisotropy in the same inferior frontal region (r=–0.76, p=0.02).