Bipolar affective disorder is a severe, chronic, life-threatening illness
(1). Epidemiological data from adolescents
(2) suggest that the prevalence of juvenile-onset bipolar disorder may be as common as rates reported in adult populations, although estimates in school-age children vary widely
(3). A number of investigators have reported that nonclassical presentations of bipolar illness, such as chronic mixed states
(4) and rapid cycling
(5), may be more common in juvenile-onset bipolar disorder than in adult bipolar disorder
(6). Even more controversial is the notion of “subsyndromal” bipolar disorder
(2). This group (i.e., with subsyndromal bipolar disorder) has been defined as “experiencing a distinct period of abnormally and persistently elevated, expansive, or irritable mood (i.e., the core manic mood symptoms) without meeting full DSM-IV criteria for bipolar disorder”
(2). The National Institute of Mental Health (NIMH) Research Roundtable on Prepubertal Bipolar Disorder
(7) agreed on the possible existence of other phenotypic possibilities that do not meet diagnostic criteria for prepubertal bipolarity but merit examination as forme fruste conditions. The term “bipolar disorder not otherwise specified” was recommended as a working diagnosis for the non-DSM-IV phenotype
(7). However, the concern remains that any relaxation of the DSM-IV bipolar criteria may risk increasing the number of false positive diagnoses.
In our study, we diagnosed a group of pediatric inpatients with intermittent explosive disorder on the basis of the occurrence of discrete episodes of failure to resist aggressive impulses, resulting in serious assaultive acts. Recurrent aggressive episodes did not have intercurrent depressive symptoms, nor did they meet minimal criteria for duration under current DSM-IV guidelines for a manic/hypomanic or major depressive episode. Furthermore, a precipitating psychosocial stressor or a diagnosis of attention deficit hyperactivity disorder (ADHD) or conduct disorder (e.g., only two patients in this group also received a diagnosis of conduct disorder) could not account for this degree of aggressiveness. Children with a diagnosis of intermittent explosive disorder have symptomatic overlap with classically defined bipolar disorder, which creates diagnostic confusion
(8). Regardless of the underlying diagnosis, several of these children’s target symptoms could potentially be treated with a mood stabilizer
(9), but it is important to clarify diagnosis before initiating treatment. This study’s goal was to help develop a set of biological markers that would differentiate these two groups.
Myo-inositol is a molecular component of an intracellular second-messenger system in which activated receptor-ligand complexes stimulate the turnover of inositol-containing phospholipids
(12). The ability of neurons to maintain a steady-state supply of cytosolic
myo-inositol appears to be crucial to the resynthesis of phosphoinositides and, conceivably, to the membrane receptor response to stimulation and neuronal homeostasis
(12). Abnormalities of this system have been shown in platelet membrane phosphoinositides of manic
(13) and lithium-treated patients with bipolar disorder
(14), suggesting a role for this secondary messenger in the neurobiology of bipolar disorders.
Method
Subjects
The UCLA Human Subjects Protection Committee approved this study. After complete description of the study, all parents and children signed a written informed consent form. Twenty unrelated patients were sequentially recruited from UCLA Neuropsychiatric Institute inpatient units and the outpatient pediatric psychopharmacology clinic over a 2-year period. All patients were administered a structured interview with the NIMH Diagnostic Interview Schedule for Children
(16) for the assessment of present and past-year axis I psychopathology. Diagnostic criteria for mania and hypomania were confirmed with third-party informants (i.e., schoolteachers, counselors, and therapists) in all participants before enrollment. All children were interviewed by a board-certified psychiatrist (P.D.), who reviewed the inpatients’ team assessment and structured interview scores with a board-certified child psychiatrist (J.M.) to reach a best-estimate diagnosis. Patients with bipolar disorder were required to have Young Mania Rating Scale
(17) scores above 12 before enrollment. Medical and psychiatric histories were taken from all patients; patients also underwent complete physical and neurological examinations and laboratory and thyroid screenings. The patients were excluded from the study if their ages were below 5 or over 18 or if they met diagnostic criteria for mental retardation, pervasive developmental disorder, schizophrenia, posttraumatic stress disorder, or Tourette’s syndrome. They were also excluded if they had active medical or neurological disease, metallic implants, or significant claustrophobia. One patient with well-controlled diabetes was allowed to participate in the study.
Ten patients met criteria for bipolar disorder (age: mean=9.8, SD=2.0), and 10 patients met criteria for intermittent explosive disorder (age: mean=9.6, SD=3.0). The study groups underwent scanning during the first week of hospitalization (four with bipolar disorder, three with intermittent explosive disorder) or while they were considered for inpatient hospitalization due to severity of illness (six with bipolar disorder, seven with intermittent explosive disorder). A small number of patients in both groups were taking medication at the time of their scan: stimulants (two with bipolar disorder, four with intermittent explosive disorder), α2 agonists (one with bipolar disorder, two with intermittent explosive disorder), divalproex sodium (two with bipolar disorder, two with intermittent explosive disorder), and risperidone (two with bipolar disorder). None of the children received sedation before scanning. The parent or parents of the children were allowed to remain in the scanner room with their children during the procedure.
A normal comparison group unmatched by age (N=13; age: mean=11.7, SD=3.6) and gender was recruited from the community and screened by a board-certified psychiatrist (P.D.) with a mental status examination and the Primary Care Evaluation of Mental Disorders: Patient Health Questionnaire
(18).
Diagnostic Features of Bipolar Disorder
Of the 10 children with bipolar disorder, four had euphoria as their predominant mania symptom. The remainder of the bipolar disorder group had irritability as their predominant mania symptom. All of the children with bipolar disorder met the criteria for bipolar disorder type I; in their most recent episode, seven were manic and three were mixed. One patient with manic bipolar disorder I had a rapid-cycling specifier, and one subject with mixed bipolar disorder type I had a psychotic specifier. Five of the patients with bipolar disorder (three with mania, two with mixed features) and three patients with intermittent explosive disorder had never been treated with medication. The brains of seven patients with bipolar disorder were scanned while they were in the midst of a manic episode; three were scanned while they were in partial remission of a manic episode. No patients underwent scanning during the depressed phase of their illness.
Diagnostic Features of Intermittent Explosive Disorder
Of the 10 children with intermittent explosive disorder, seven had recurrent explosive episodes without intercurrent depressive or manic symptoms (patients 1, 2, 4, 5, 6, 9, and 10); three had alternations (over days) between explosive and dysphoric symptoms that did not meet minimal criteria for a manic or major depressive episode (patients 3, 7, and 8). One of the associated features of intermittent explosive disorder in these children was the presence of impulsivity and aggressive behavior between explosive episodes. The disorder in all of these children resulted in severe difficulties with interpersonal relationships, school suspension, and psychiatric hospitalization.
1H MRS Protocol
All patients and comparison subjects underwent scanning with a GE 1.5-T Signa scanner (General Electric, Milwaukee). Landmarks of the axial plane were noted at the center of the forehead, 1 cm above the eyebrows, in all of the subjects to standardize heads positioning from scan to scan. To minimize head movements, the forehead was affixed with adhesive tape to a magnetic resonance imaging stretcher, and neck support was provided as necessary. Scanning was repeated when motion artifacts were detected on a localizing scan or when the spectral line clearly revealed a motion artifact (from subject movement, distortion, or broadening). If necessary, those scans were interrupted and reinitiated after head adjustments were made and further head support was offered. A short series of axial-localizing images were acquired (with spin echo, TR/TE=500 msec/8 msec, 4-mm contiguous slices, 256×192 matrix size, number of excitations=1, field of view=26 cm, acquisition time <2 minutes) to maximize differentiation of gray/white matter and to measure the specific regions of interest. Single-voxel localization was achieved by using a PRESS sequence. For each spectrum, 64 water-suppressed and four unsuppressed water signals were acquired (TR/TE=3 seconds/30 msec, total acquisition time=3.5 minutes).
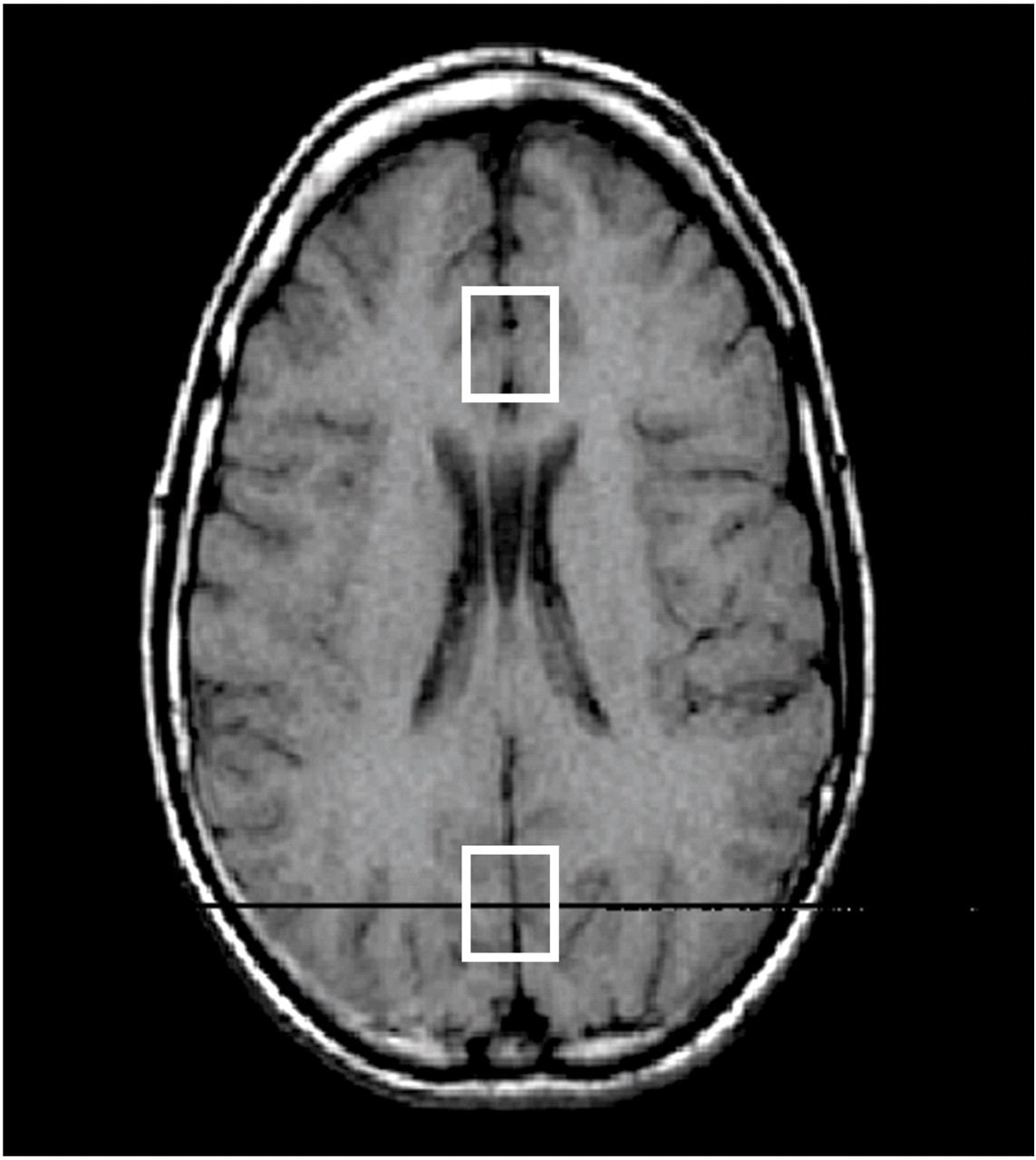
A systematic approach to positioning reference voxels to identifiable anatomical landmarks in all subjects was based on a human brain reference atlas
(19). An axial cut approximately 1 cm above the genu of the corpus callosum, showing a continuous view of the anterior and posterior horns of the lateral ventricles, was chosen as a reference slice (
Figure 1). The center of an 8-cc voxel of predominantly gray matter was centered on the prefrontal interhemispheric fissure, distal to the anterior horns of the lateral ventricles. Its proximal quadrant was placed immediately adjacent to the zone of delimitation between white and gray matter (i.e., the corpus callosum’s margin with the cingulate gyrus), in an area partially occupying prefrontal rostral Brodmann’s areas 24 and 32
(20). This voxel placement was selected according to anatomical correlation with pathways postulated in the neurobiology of cognitive-affective components of human behavior
(21) and mood disorders
(22).
We also placed a control voxel in the occipital cortex (
Figure 1), a cortical area purportedly not involved in the neurobiology of mood disorders
(23). On axial cuts, an investigator (P.D.) identified the internal cerebral veins and the thalamus (bilaterally) in order to ensure the inclusion of a cortical area demarcated above the cerebellum. A 5-mm cut above this landmark constituted the lower plane of the 2×2×2 cm
3 occipital control voxel. Its anterior border was placed at least 5 mm proximal to the posterior horns of the lateral ventricle in order to avoid including the parahippocampal gyri. One cm above the lower plane, the center of the voxel fell within a cortical area occupied mostly by the occipital striate area and occipital gyri, comprising portions of Brodmann’s areas 18, 19, and 23
(19), and anatomically corresponding to the calcarine sulci and cuneus
(19). One and one-half cm above the lower plane, the control voxel included most of the former structures and a few interhemispheric vessels (i.e., the straight sinus). Only at 2 cm above the lower plane, concordant with the partial effacement of the posterior horns of the lateral ventricles on the axial cut, the superior plane of the control voxel included the beginning of the lower portion of the superior parietal lobes, in addition to the occipital areas described.
1H MRS Processing
Data collection and analyses were conducted and interpreted in a manner that was blind to group membership by a research assistant (K.Y.) who was trained in
1H MRS acquisition and spectral analysis. The raw
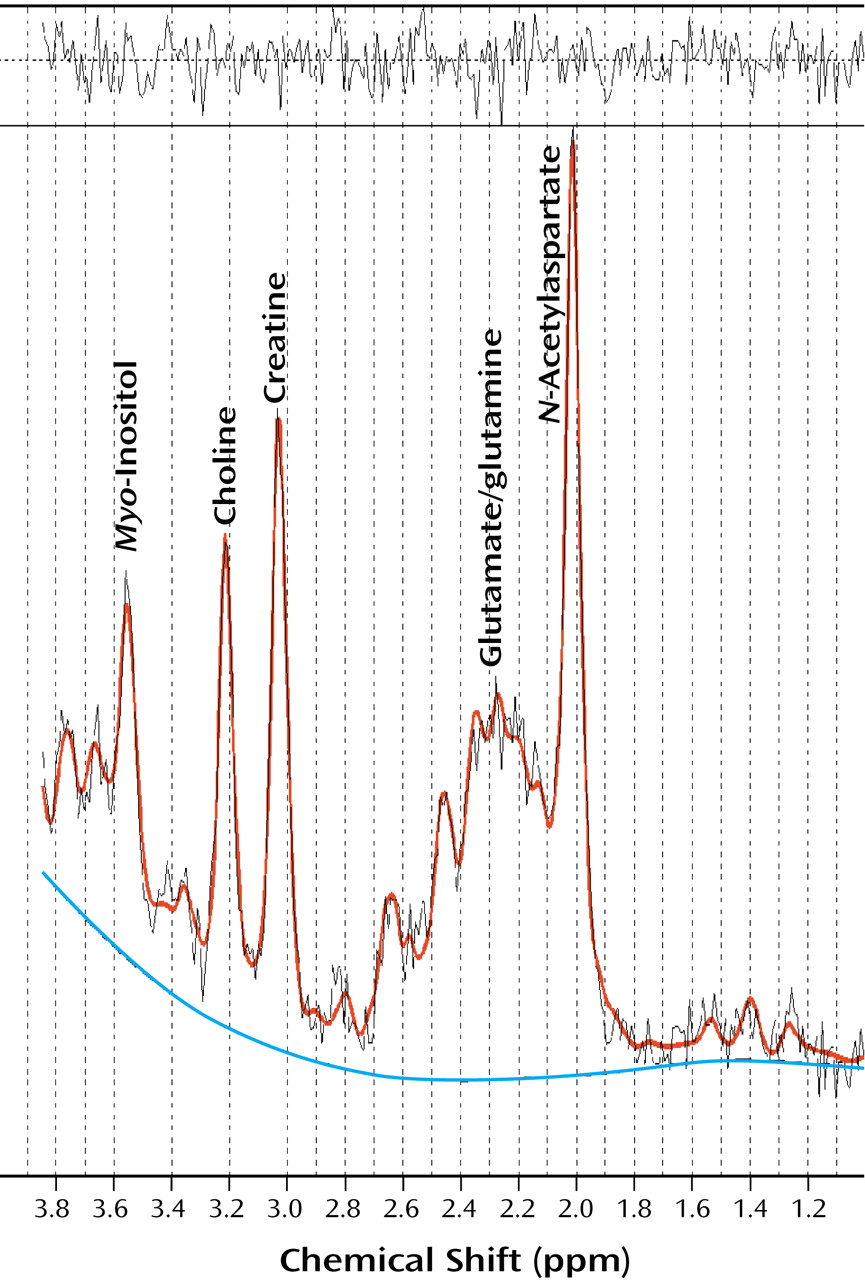
1H MRS data were processed by using an offline workstation. All spectra were processed with the LC-Model
(24) (
Figure 2), an operator-independent fitting routine. The water-suppressed time domain data were analyzed at between 1.0 ppm and 4.0 ppm without further T
1 and T
2 correction. The basis data set provided by the vendor
(24) was used and then scaled to a consistent transmitter gain. Absolute values of
N-acetylaspartate, creatine-phosphocreatine, choline moieties, and
myo-inositol are reported in millimol/liter that are uncorrected for T
1 and T
2 saturation. The signal from the tissue water was also acquired from the same location that was used for the acquisition of metabolite signals. The unsuppressed water signal was used for eddy current compensation and also for absolute quantitation. The water-suppressed
1H MRS data were recorded by using the PRESS sequence (8-ml voxel, TR=3 seconds, TE=30 msec, 64 averages of water-suppressed metabolite signals, and four averages of unsuppressed water signals). The raw
1H MRS data were transferred to a workstation and processed by using the LC-Model. They were not corrected for brain atrophy (in the CSF) or compartmentalization of gray and white tissue. Ratios were calculated with respect to creatine-phosphocreatine levels.
The reliability of measured data was controlled by periodic calibration of the MR scanner against standard solutions of known concentrations for spectroscopy. The specific bandwidth of water suppression of our subjects was 52 to 65 Hz. We calculated the means and standard deviations of the line widths on the spectra of our subjects. The mean line width was 7.3 Hz, and the standard deviation was 1.7 Hz. All line widths above 15 Hz were discarded and reacquired. Results were reported in ratios of creatine-phosphocreatine and absolute molar concentrations. In order to verify our assumption that creatine-phosphocreatine levels would not vary between patients and comparison subjects, we measured ratios of creatine-phosphocreatine to water for both groups and found that the variation was only 1.8%.
Data Analysis
Comparisons of metabolic resonance across groups (bipolar disorder versus intermittent explosive disorder and bipolar disorder versus normal comparison) were initially assessed by using Wilcoxon rank sum tests to address possible nonnormality of the distribution of outcome scores. Comparisons between groups were made from similar regions, i.e., the anterior cingulate cortex versus the anterior cingulate cortex and likewise for the occipital cortex. Subsequent analyses compared results by using analysis of variance (ANOVA) with a Tukey test to correct for multiple comparisons, as well as regression analysis to control for age. Two different models were explored for the contribution of age: one that entered age as a continuous predictor and another in which two indicator-variable predictors were used to reflect a partition of age into three categories (<9, 9–11.5, >11.5). Three additional comparative models of analyses were explored (model 1 includes age and gender as covariates, model 2 includes age only, and model 3 includes gender only). Medication status was not considered as a covariate because of the small number of medication classes used in this small group.
Pearson’s correlation coefficients between myo-inositol (mmol/liter) and ratings of mania (scores on the Young Mania Rating Scale) were evaluated separately within the two diagnostic groups (bipolar disorder and intermittent explosive disorder) and in the pooled group. Differences between groups in the regression coefficients of the rating scales for myo-inositol (mmol/liter) were evaluated by testing the interaction term in multiple regression models that included group, score on the rating scale, and their interaction as independent variables, with myo-inositol (mmol/liter) measure as the dependent variable.
The Pearson correlation coefficients between myo-inositol (mmol/liter) and ratings of mania (scores on the Young Mania Rating Scale) were calculated with SAS (SAS Institute, Cary, N.C.). The remaining analyses (i.e., regression analysis, ANOVA) were calculated by using SPSS (SPSS, Chicago).
Results
Demographic characteristics of the groups and scores on the Young Mania Rating Scale are displayed in
Table 1. More boys than girls underwent scanning, and the age distribution shows more preadolescents than adolescents with both bipolar disorder and intermittent explosive disorder. Nine patients with bipolar disorder were Caucasian, and one was of mixed ethnicity. Nine patients with intermittent explosive disorder were Caucasian, and one was of mixed ethnicity. The mean number of medications taken at the time of scanning was 0.9 (SD=1.1) per patient with bipolar disorder and 0.9 (SD=0.7) per patient with intermittent explosive disorder. Four children with intermittent explosive disorder had a Young Mania Rating Scale score above 12 (the estimated cutoff for adult mania) at the time of scanning, whereas all children with bipolar disorder had a Young Mania Rating Scale score above 12 at the time of scanning. Comorbidities are noted on
Table 1. Eight of the 10 patients with bipolar disorder were diagnosed with comorbid ADHD, nine with oppositional defiant disorder, and seven with conduct disorder. In comparison, seven of the 10 patients with intermittent explosive disorder were diagnosed with comorbid ADHD, nine with oppositional defiant disorder, and only two with conduct disorder.
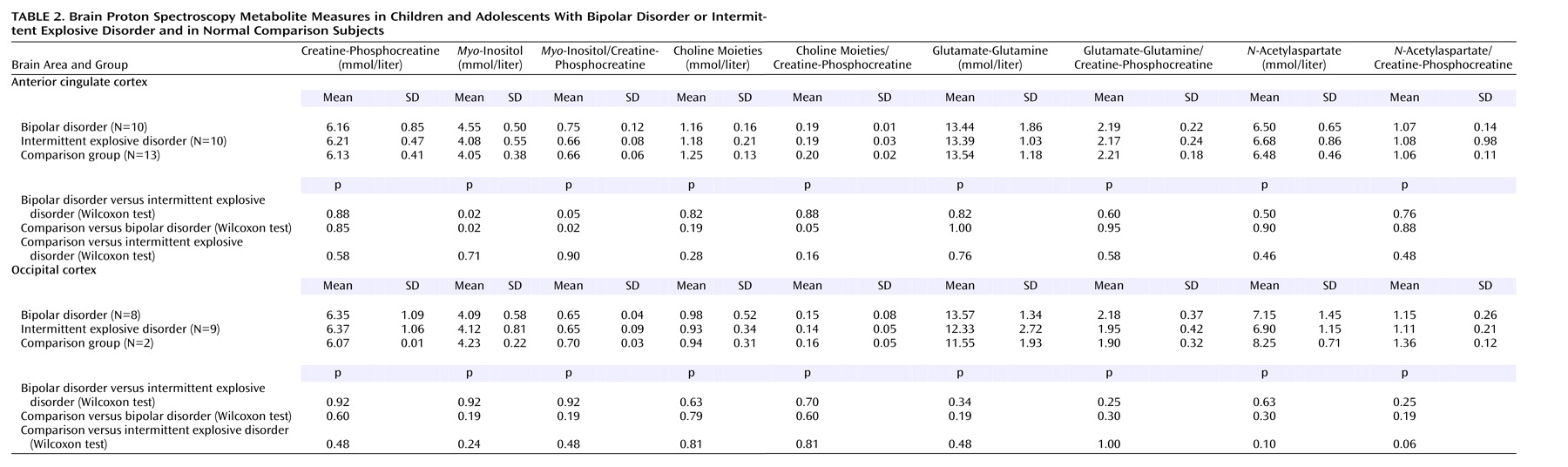
Table 2 shows the results of prefrontal anterior cingulate cortex and occipital cortex
1H MRS metabolite measures reported in absolute millimolar concentrations (mmol/liter) and creatine-phosphocreatine ratios. The patients with bipolar disorder had significantly higher mean anterior cingulate
myo-inositol (mmol/liter) and
myo-inositol/creatine-phosphocreatine measures than the patients with intermittent explosive disorder (p<0.02) and the normal comparison subjects (p<0.02). There were no significant differences in anterior cingulate
myo-inositol (mmol/liter) or
myo-inositol/creatine-phosphocreatine levels between the patients with intermittent explosive disorder and the normal comparison subjects. There was also a significantly higher anterior cingulate choline moieties/creatine-phosphocreatine measure (p=0.05) in the normal comparison subjects than in the patients with bipolar disorder. There was no significant difference between groups for any measure of metabolite in the occipital cortex.
Results were similar when analyzed with an ANOVA by means of Tukey’s multiple-comparisons test. The ANOVA F test comparing means across the three groups resulted in significance (p<0.04). The pairwise comparison of means with the Tukey test again showed a significant difference between the group with bipolar disorder and the comparison group (95% confidence interval [CI]=0.01–0.10) and a nearly significant difference between the groups with bipolar disorder and intermittent explosive disorder (95% CI=0.05–0.10). In regression analyses, when age was entered as a continuous predictor, the difference between the group with bipolar disorder and the normal comparison group remained significant (beta=0.55, p<0.02); the difference between the group with bipolar disorder and the group with intermittent explosive disorder reached significance (beta=0.47, p<0.04). When regression analysis was conducted with the age effect represented by indicators for age category, the results were similar, but the difference between the group with bipolar disorder and the group with intermittent explosive disorder was also nearly significant (bipolar disorder versus normal comparison: beta=0.50, p=0.02; bipolar disorder versus intermittent explosive disorder: beta=0.42, p<0.07).
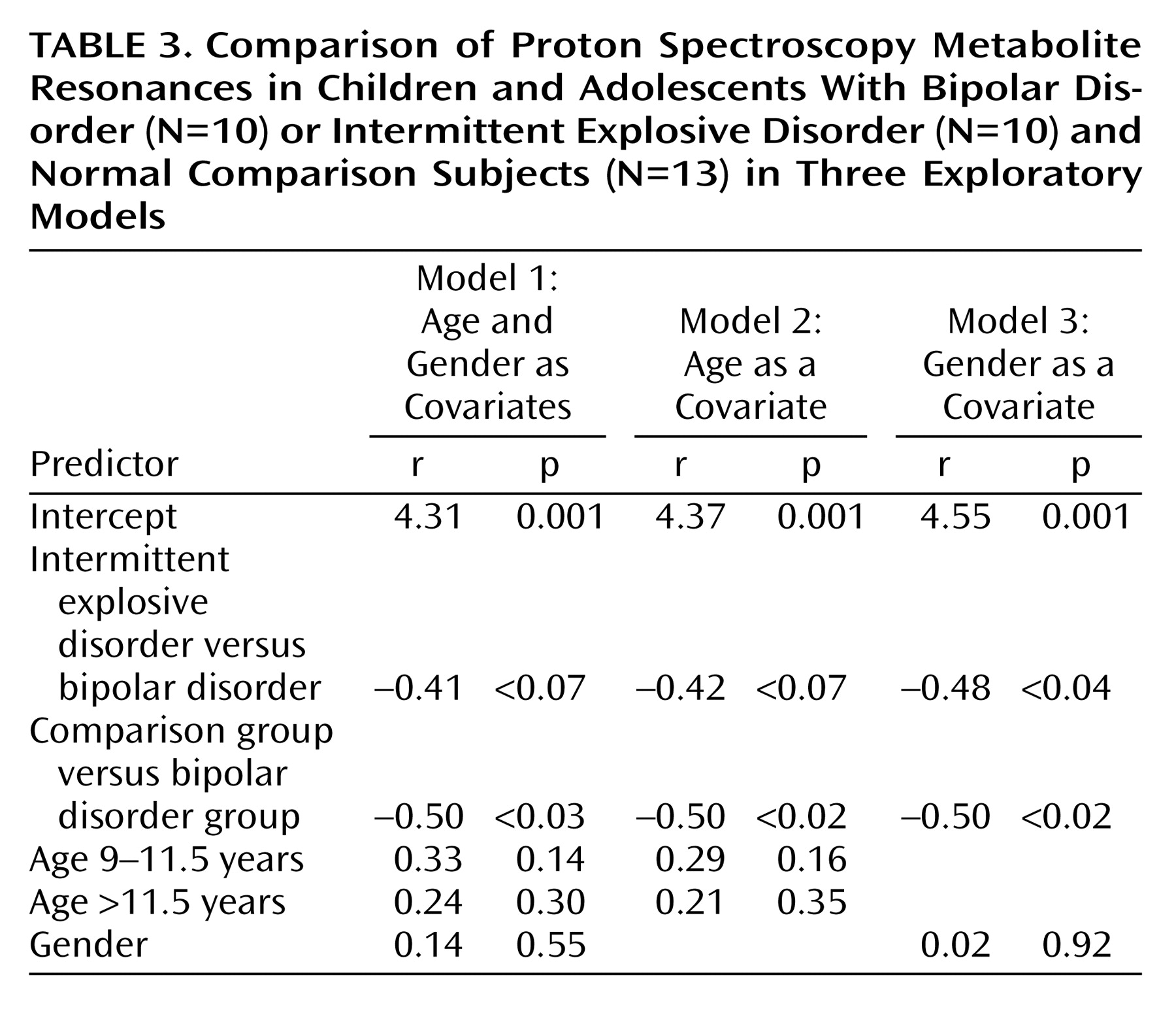
The level of significance was not modified for the comparison between the patients with bipolar disorder and the normal comparison subjects in any of the three exploratory models of analyses (
Table 3). The comparison between the patients with bipolar disorder and the patients with intermittent explosive disorder was modified according to the model of analysis used. With model 1 (a covariate analysis including both age and gender) and model 3 (a covariate analysis including age only), a comparison between the patients with bipolar disorder and the patients with intermittent explosive disorder resulted in p values of 0.07 and <0.61, respectively, and a comparison with model 3 (a covariate analysis with gender only), resulted in significance (p<0.04). Correlations between the measures of anterior cingulate
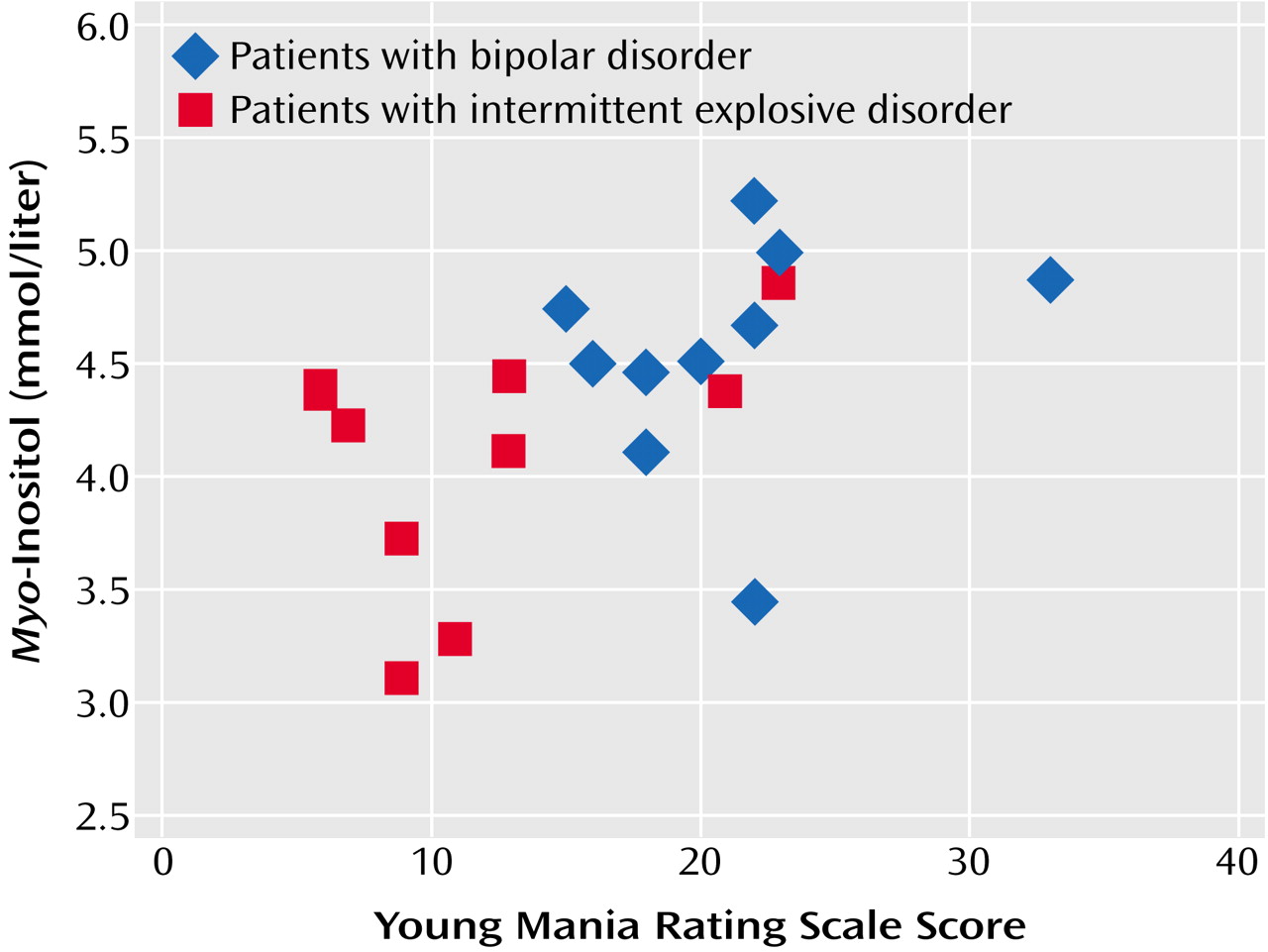
myo-inositol (mmol/liter) and Young Mania Rating Scale scores were r=0.23 for the patients with bipolar disorder, r=0.41 for the patients with intermittent explosive disorder, and r=0.51 (p=0.02) for the pooled group. The Young Mania Rating Scale correlated significantly with
myo-inositol (mmol/liter) in the pooled sample (r=0.51, p=0.02) but not in either group taken separately, as illustrated in
Figure 3. The attenuation of the within-groups correlations was because the two groups scored almost completely differently on the Young Mania Rating Scale, resulting in a restriction of range on that scale. Higher
myo-inositol (mmol/liter) values correlated (in the pooled group) with higher scores on the Young Mania Rating Scale scores, corresponding mostly with the group with bipolar disorder, while lower Young Mania Rating Scale scores correlated with lower
myo-inositol (mmol/liter) values, corresponding overall with the group with intermittent explosive disorder.
Discussion
Studies of in vivo
1H MRS have traditionally included results in ratios of creatine-phosphocreatine, with this peak as a reference against compounds of interest
(25). However, because of potential variability of creatine-phosphocreatine levels dependent on physiological changes or the influence of medication
(26), we reported results in both ratios of creatine-phosphocreatine and estimated absolute brain concentrations (mmol/liter) and noted parallel group differences, regardless of metabolite measurement approach.
Our data indicated significantly higher anterior cingulate
myo-inositol (mmol/liter) and
myo-inositol/creatine-phosphocreatine measures in children with bipolar disorder than in normal comparison subjects and children with intermittent explosive disorder, thus confirming our hypothesis. This finding was not seen in the occipital cortex, suggesting that
myo-inositol (mmol/liter) and
myo-inositol/creatine-phosphocreatine measurement changes may be regionally specific to the anterior cingulate cortex, which is a component of a limbic-thalamic-prefrontal cortical circuit involved in mood regulation
(23). Our current findings in a new group of children with bipolar disorder replicate those in our previous study of differences between patients with bipolar disorder and normal comparison subjects
(15). To our knowledge, this study is the first attempt at demonstrating a specific biological differentiation between juvenile-onset bipolar disorder and an overlapping clinical phenotype by means of in vivo
1H MRS.
Our findings are consistent with the report by Sharma et al.
(27) of four adults with bipolar disorder and treated with lithium who had elevated
myo-inositol/creatine-phosphocreatine measures in the basal ganglia. Our data are not consistent with a previous
1H MRS study of 10 children with bipolar disorder, ages 6 to 12 years
(28), that showed higher glutamate-glutamine levels in the frontal lobes and basal ganglia than a normal comparison group. However, the regions of interest in that study and ours are not entirely comparable, since Castillo et al.
(28) used 3×3×3 cm
3 voxels placed in a region that included the dorsolateral and anterior cingulate prefrontal cortex, whereas we used placement of a 2×2×2 cm
3 voxel in the anterior cingulate cortex in our study.
Our data are also nonconcordant with a
1H MRS study of the anterior cingulate cortex of nine adults with bipolar disorder who were taking either lithium or divalproex sodium in relation to normal comparison subjects
(29). In that study, no differences in
myo-inositol/creatine-phosphocreatine measures were observed between patients with bipolar disorder and normal comparison subjects, but the choline moieties/creatine-phosphocreatine measures were significantly higher in the right cingulate cortex of the adult subjects with bipolar disorder than in the comparison subjects. However, medication status and serial assessments at different states of the illness may have constituted an important confounder in that study
(29).
The mechanism of action resulting in higher
myo-inositol (mmol/liter) concentrations in patients with bipolar disorder than in normal comparison subjects and patients with intermittent explosive disorder is unknown. However, one potential explanation involves
myo-inositol’s role as an osmolar agent
(30), primarily in glial cells
(31). Hyperosmolarity triggering the accumulation of
myo-inositol has been described in glioma cells
(32) and in humans with hyponatremia
(33), renal failure, and alcohol-induced hyperosmolarity
(34). We speculate that a similar phenomenon may occur during a manic episode in youth with bipolar disorder, in which extracellular hyperosmolar conditions could trigger changes leading to alteration in the concentrations of intracellular organic solutes, specifically
myo-inositol
(31), in glial cells in the anterior cingulate.
The relevance of metabolic changes in the anterior cingulate is supported by several brain-activation studies (with positron emission tomography) involving adult
(22) and pediatric
(35) patients with bipolar disorder and showing differential activation induced by mood states and drug treatment in brain regions adjacent to the anterior cingulate cortex. The anterior cingulate cortex, a large cortical region around the rostrum of the corpus callosum
(36), has extensive connections with the amygdala, a limbic nuclear structure with a pivotal role in affect regulation
(23). Disruption of this cortical circuit may contribute to the behavioral syndromes associated with juvenile-onset bipolar disorder.
Young Mania Rating Scale scores correlated significantly with myo-inositol (mmol/liter) levels in the pooled study group. Attenuation of the within-groups (bipolar disorder and intermittent explosive disorder) corrections may be partly due to the strain of achieving a statistical correlation within smaller groups. However, these scores may be statistically correlated with anterior cingulate myo-inositol levels (mmol/liter) in each group in larger samples, since higher myo-inositol (mmol/liter) measures correlated with higher scores on the Young Mania Rating Scale, which corresponded mostly with the group with bipolar disorder, while lower scores on the Young Mania Rating Scale correlated with lower myo-inositol (mmol/liter) measures, which corresponded overall with the group with intermittent explosive disorder.
A potential confounder in our study could be the diagnosis of ADHD, a comorbid condition in 80% of the patients with bipolar disorder and 70% of the patients with intermittent explosive disorder. Variations in brain morphology
(37–
42) and possible
1H MRS
N-acetylaspartate/creatine-phosphocreatine
(43,
44) and choline moieties/creatine-phosphocreatine
(43) measurement abnormalities have been described in patients with ADHD. Some controversy remains regarding the specificity of brain structural abnormalities in children with ADHD
(45). However, in reference to our study, it seems unlikely that these factors would play a major role in data analyses since the regions of structural abnormalities identified in the literature are distinct from the regions we examined. Additionally, while
N-acetylaspartate/creatine-phosphocreatine and choline moieties/creatine-phosphocreatine measurement abnormalities in the bilateral striatum
(43) was associated with children with ADHD and
N-acetylaspartate/creatine-phosphocreatine measurement abnormalities in the dorsolateral prefrontal cortex were associated with adults with ADHD
(44), there were no apparent effects on
myo-inositol levels in either of these studies.
Psychotropic medications may constitute an additional source of variance in the analyses of human
1H MRS. Although definitive data are lacking, two current studies have examined the potential effect of psychotropic medications on
1H MRS. A
1H MRS study
(43) showed that 10 mg/day of methylphenidate given to previously untreated boys diagnosed with ADHD did not affect the measures of
N-acetylaspartate, choline moieties/creatine-phosphocreatine,
myo-inositol/creatine-phosphocreatine, or glutamate-glutamine/creatine-phosphocreatine in the globus pallidus, suggesting that methylphenidate does not affect
1H MRS in pediatric ADHD. Conversely, Bertolino and colleagues
(46) showed that, although they were taking antipsychotics, patients with schizophrenia had significantly higher
N-acetylaspartate/creatine-phosphocreatine measurements in the dorsolateral prefrontal cortex, suggesting that antipsychotics may increase
N-acetylaspartate levels in these patients. However,
myo-inositol was not affected, and the dorsolateral prefrontal cortex was not the cortical area investigated in our study of children with bipolar disorder. Further data are needed to evaluate the possible effect that neuroleptics and other psychotropic medications may have on
1H MRS in the human brain. We acknowledge this as a limitation of our study.
Other Limitations
Uncertainty about the developmental modifications of diagnostic criteria for early-onset bipolar disorder may lessen the conclusions pertaining to biological measures in this population. Likewise, the presence of comorbid psychiatric diagnoses in both groups, the most common being ADHD
(4), may be a potential source of variability in our results, restricting their generalizability.
One subject with bipolar disorder and well-controlled diabetes was included in the study group. While a
1H MRS study of adult patients with diabetes mellitus (including episodes of ketoacidosis)
(47) discovered higher average parietal white and gray matter measurements of glucose,
myo-inositol, and choline moieties than in healthy subjects, differences in regions of interest, phase of the illness, and age suggest that this was less likely to significantly affect the results in our subject.
Lack of CSF segmentation was a limitation of our study. Although there are data suggesting that precise composition of white and gray matter and CSF must be known to avoid partial-volume effects in single-voxel
1H MRS
(48), which may lead to underestimation of brain metabolite concentrations
(49), acquisitions with short echo time with in vivo PRESS
1H MRS (T
2, TE=35 msec) fitted into the time domain with linear combination modeling allowed us to use the Lorentzian robust-minimization procedure (referred to as maximum-likelihood or an m-estimate fitting) to accurately quantify metabolite concentrations. The expected impact of correcting for CSF and relaxation values was therefore attenuated with the use of linear combination modeling and comparisons between data from groups acquired from similar regions, i.e., within the anterior cingulate
(27).
It has been reported that at 1.5 T field strength, the
1H MRS peak due to the glycine spectrum overlaps with the
myo-inositol peak at 3.54 ppm
(50). We acknowledge, therefore, the potential confounding presented by the resonances of glycine and inositol-1-phosphate at the
myo-inositol resonance peak. Nevertheless, in concordance with Moore and colleagues
(14,
51), we believe that these compounds contribute a minor (<5%) component to the
myo-inositol resonance measurement. Additional limitations of the study include the small group size and the paucity of data concerning a possible developmental variability in metabolite resonances across childhood and adolescence
(52).
Clinical Implications
Differentiation of multiple comorbid psychiatric disorders in the clinical setting is often a challenge. Furthermore, the parents of children with bipolar disorder or intermittent explosive disorder often oppose the idea of a trial with a mood stabilizer for the treatment of target symptoms unless a diagnosis of bipolar disorder has been demonstrated by clinical assessment or other measures. Our findings may help clinicians anchor a component of a cross-sectional assessment of children who have overlapping symptoms (of severe impulsivity, irritability, and mood liability) and provide guidance in choosing proper medication. This is of particular importance since data are lacking to support the use of mood stabilizers in children and adolescents with intermittent explosive disorder, as well.
In summary, this study presents evidence of possible differences in
myo-inositol levels among a group of children with bipolar disorder and a group with intermittent explosive disorder and normal comparison subjects. These data strongly suggest that elevated
myo-inositol levels may reflect a key neurobiological feature of juvenile-onset bipolar disorder in the manic phase of the illness. Our results offer construct validity to the categorical distinction between these two groups, as defined by DSM-IV. Although our group with intermittent explosive disorder may resemble the adolescent group with subsyndromal bipolar disorder described by Lewinsohn et al.
(2) or the group with bipolar disorder not otherwise specified that was designated by the NIMH Research Round on Prepubertal Bipolar Disorder
(7), we make no assumptions about their phenotypic relationship to bipolar spectrum disorders. The intermittent explosive disorder described in this study is prevalent among adults
(53–
55) and possibly children and adolescents
(56). Our finding, if replicated, could contribute to a biological differentiation of trait-related differences between these two pediatric disorders with symptomatic overlap. Such an approach could provide a marker that is external to the question posed by the complicated comorbid phenotypes of individual patients, thus contributing to the validation of diagnostic categories necessary for appropriate medication.