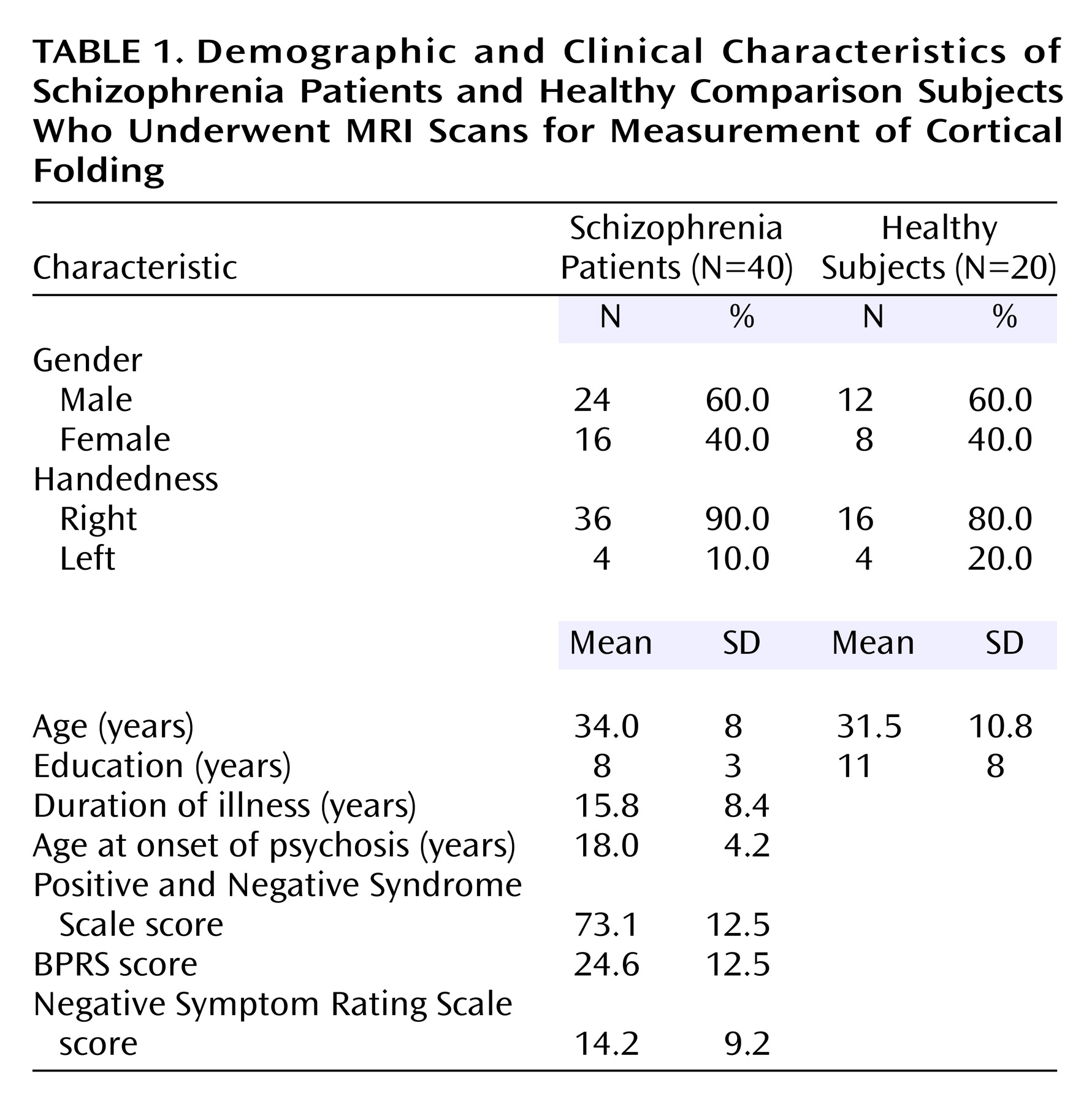
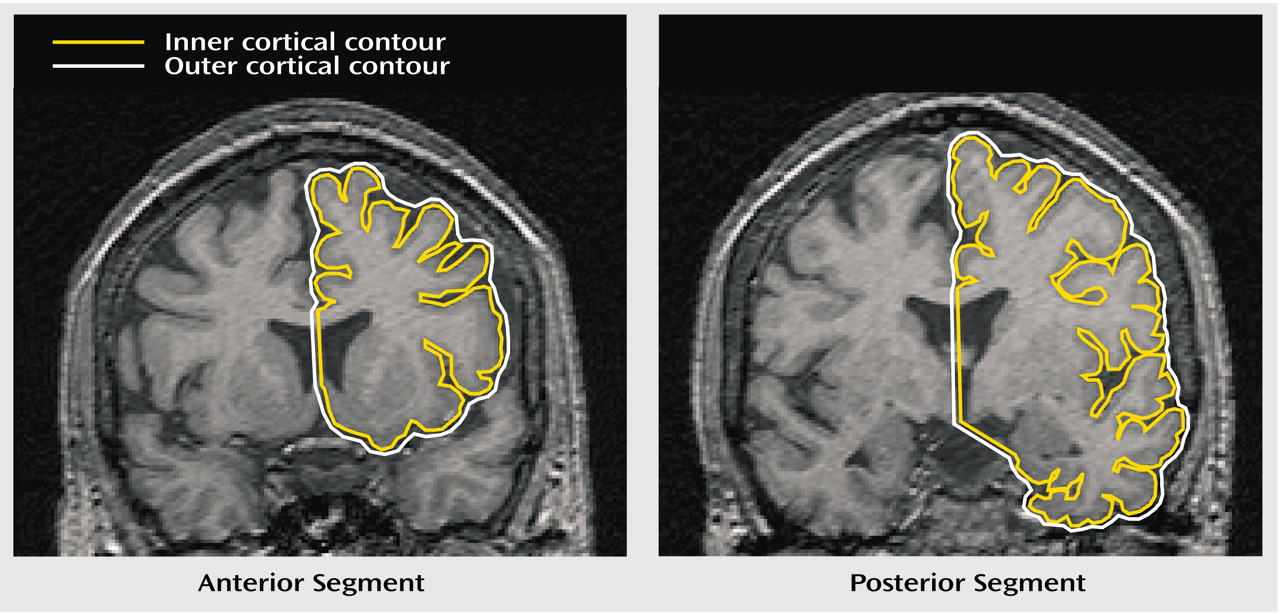
Between-Group Differences
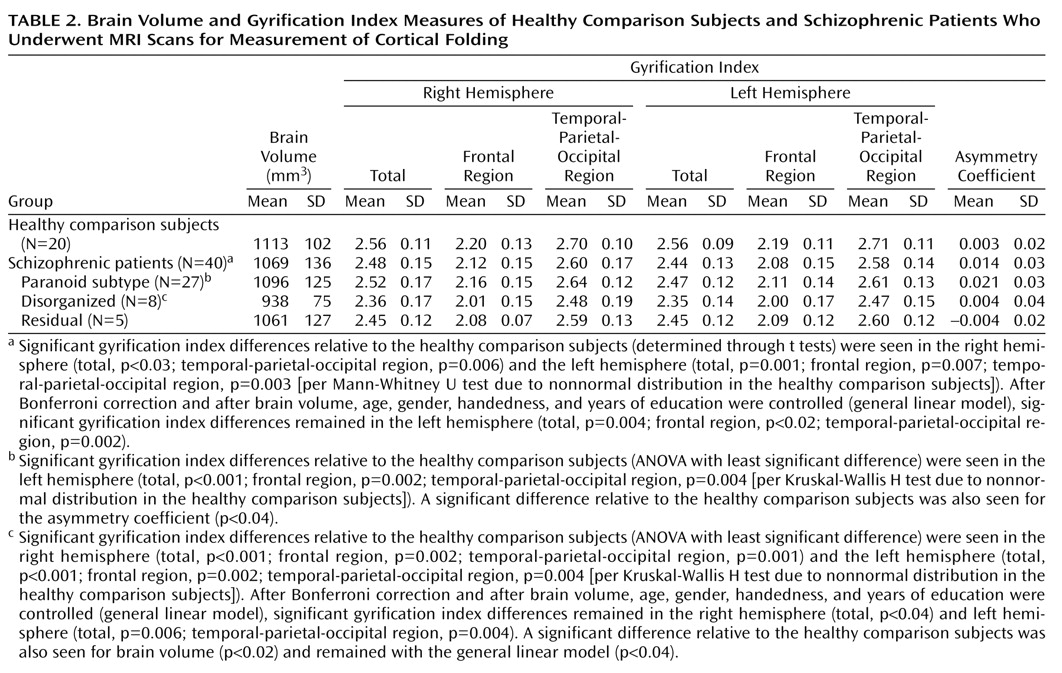
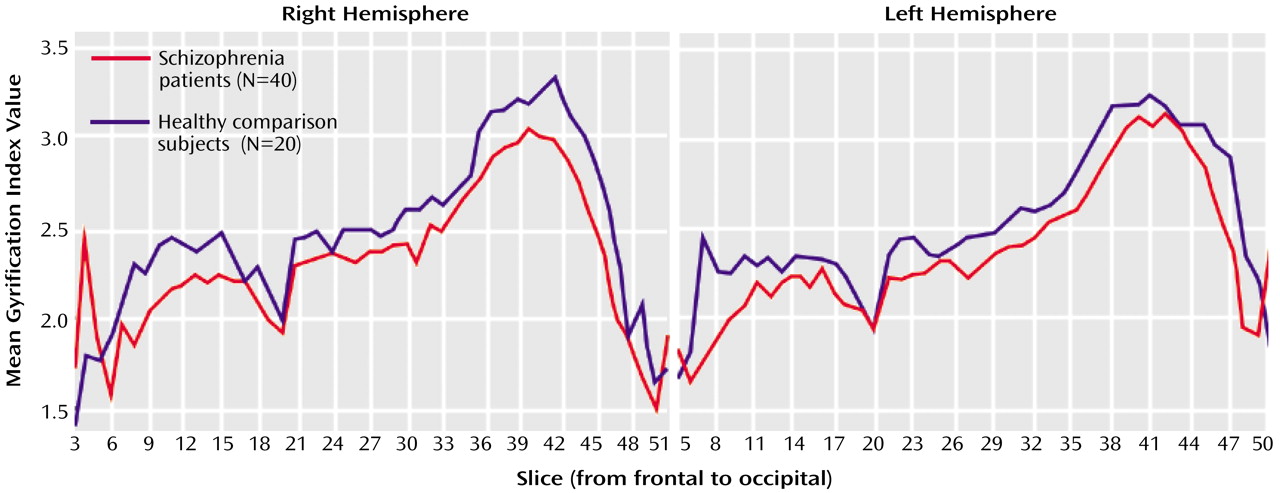
Relative to healthy subjects, schizophrenic patients showed significant left gyrification index reductions (–4.5%) and, to a lesser extent, right gyrification index reductions (–3.3%). Such findings are in agreement with the reduction of cortical folding in left hemispheres of nine male schizophrenic patients as reported by Kulynych et al.
(16). On the other hand, in a postmortem study, Vogeley et al.
(23) found a prefrontal “hypergyria” in the right hemispheres of 11 male schizophrenic patients. This finding was replicated by the same group
(17) in an in vivo MRI study with 12 patients. In the first study
(23), the calculation of the mean gyrification index of the prefrontal cortex was restricted to three sections (20 μm in thickness, intervals of 2 mm). In the second study
(17), they included three slices of similar topography as well as two other slices 20 and 10 mm anterior to the genu of the corpus callosum. Differences in method make it difficult to compare those results with the findings of our study, since we measured slices at intervals of 3.6 mm along the whole cortex (about 50 slices in each hemisphere). In order to allow a more direct comparison of our data with the postmortem study of Vogeley et al.
(23), we repeated our between-group comparison restricting the gyrification index measurement to slices 3, 4, and 5. Like these authors, we found “hypergyria” circumscribed to this right prefrontal area. There is some evidence that schizophrenic patients have the normal counterclockwise torque of the cerebral hemispheres diminished or absent in comparison with healthy individuals (with the right frontal and left occipital lobes more protruding than their counterparts in the opposite hemisphere)
(24). According to this view, the apparent right prefrontal hypergyria reported by Vogeley et al. and ourselves could be seen as an artifact of differences in the topographic positioning of the slices of patients and comparison subjects.
In our study, the left hemisphere gyrification index of the schizophrenia patients was significantly lower than the right hemisphere gyrification index. Moreover, except for the disorganized subtype, all statistically significant differences between schizophrenic subgroups and healthy comparison subjects were restricted to the left hemisphere, suggesting that the gyrification index abnormalities have a main effect of left lateralization. Supposing that the reduction of the cortical folding reflects an underlying abnormality of neuronal connectivity, as suggested by some authors
(13–
15), our results are in agreement with a vast number of studies that have shown a predominance of abnormalities in the left hemisphere of schizophrenic patients
(24,
25).
In both groups, we found significant bilateral inverse correlations of the gyrification index with age. In addition, the right gyrification index showed a positive correlation with brain volume. Such results are in disagreement with results reported by previous studies, where no significant correlations were observed between the cortical folding measures and demographic variables
(13,
16).
The overall “hypogyria” reported in the present study can be discussed in view of the theories on cortical morphogenesis. The biomechanical hypothesis
(26,
27) suggests that cortical folding results from different tensions of growth between the supragranular (layers I, II, and III) and infragranular (layers IV, V, and VI) strata. In a complementary way, the tension-based theory of cortical morphogenesis
(28) argues that the mechanical tension along with axons, dendrites, and glial processes exerts a main force in the cortical folding. During corticogenesis, the cortical laminae, initially tethered only by radial glial process, become subsequently anchored by corticocortical and corticosubcortical connections. Considering the viscoelastic properties of axons, areas with more compact corticocortical wiring (larger approximative tension) tend to get together, originating an outward fold (gyrus); on the other hand, regions with poor circuitry (lesser cohesive force) tend to stretch the neuronal fibers, giving rise to inward folds (sulcus). The subplate, a transient fetal structure, contains neuronal populations previous to the migrant neurons to which they establish transient synapses involving neurotransmitters and neuromodulators; these connections are still poorly known but are certainly important for the understanding of corticogenesis
(14). Armstrong et al.
(15) drew attention to the chronological similarity between subplate and cortical folding. The subplate changes from a bilaminar to a single structure around the 21st ontogenetic week (when the cortex begins its rapid convolutional development) and diminishes in size after birth (when the cortical folding reaches its adult values). Hence, the subplate (whose cells project primarily to middle and superficial layers of cortical plate) could provide the mechanical forces responsible for the cortical folding, formed mainly as a consequence of the corticocortical circuitry.
Previous neuropathologic findings in schizophrenia add evidence in agreement with the above model. With regard to cytoarchitectural features in schizophrenia (see Harrison
[1] for a critical review), the pyramidal neurons of the prefrontal cortex and limbic system have been shown to be smaller and more densely packed. The cortex is thinner, especially in laminae II and III, and the reduced volume in superficial layers suggests less extensive or aberrant synaptic connections formed by incoming corticocortical fibers, which are modulated by interaction with subplate cells during corticogenesis.
Gyrification Index: Relationship With Clinical Subtypes and Symptoms
The patients with the DSM-IV disorganized subtype of schizophrenia showed the smallest indexes of cortical folding. However, left gyrification index reduction was not exclusive to this subtype, since the paranoid and residual subtypes also showed left gyrification index reductions that reached statistical significance.
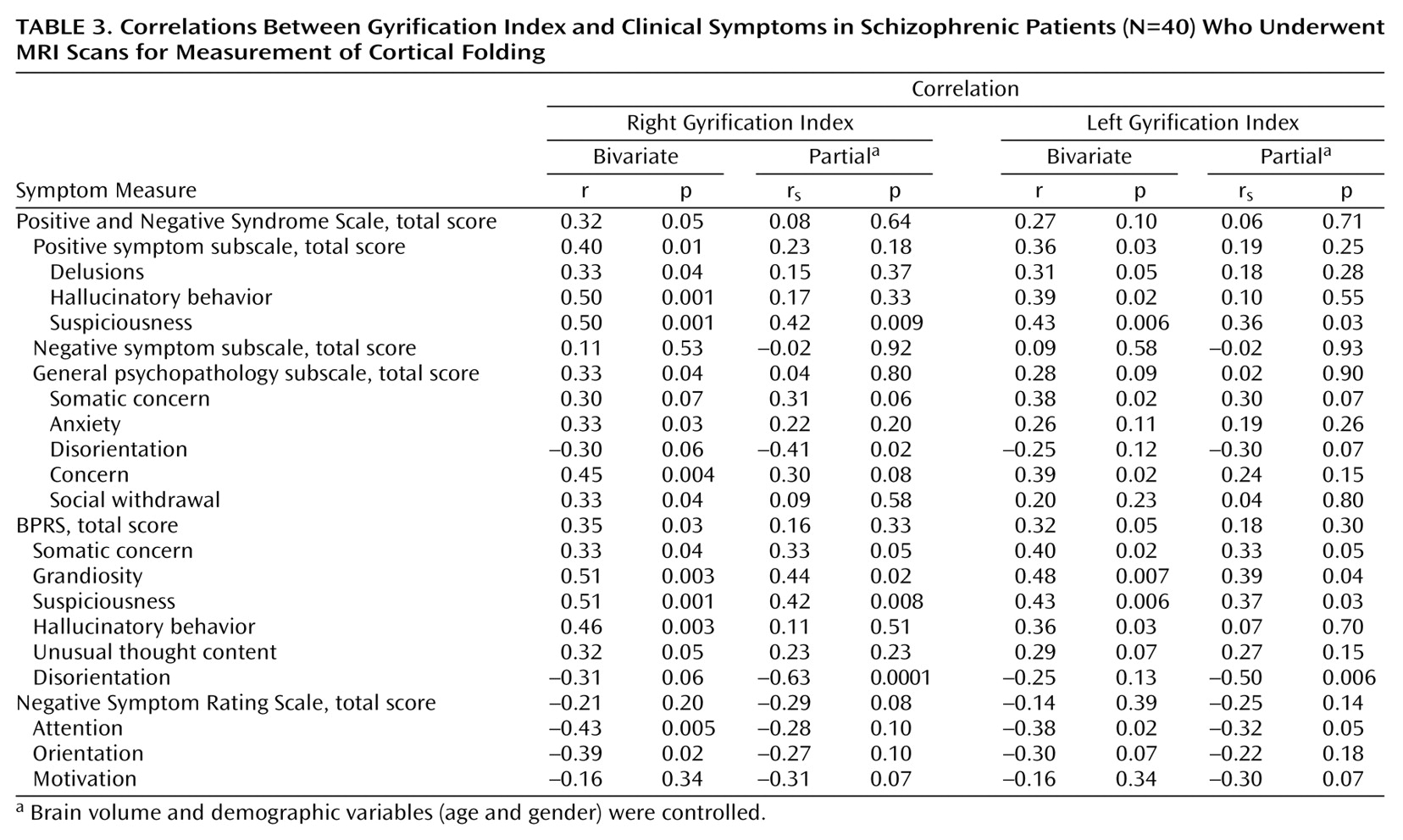
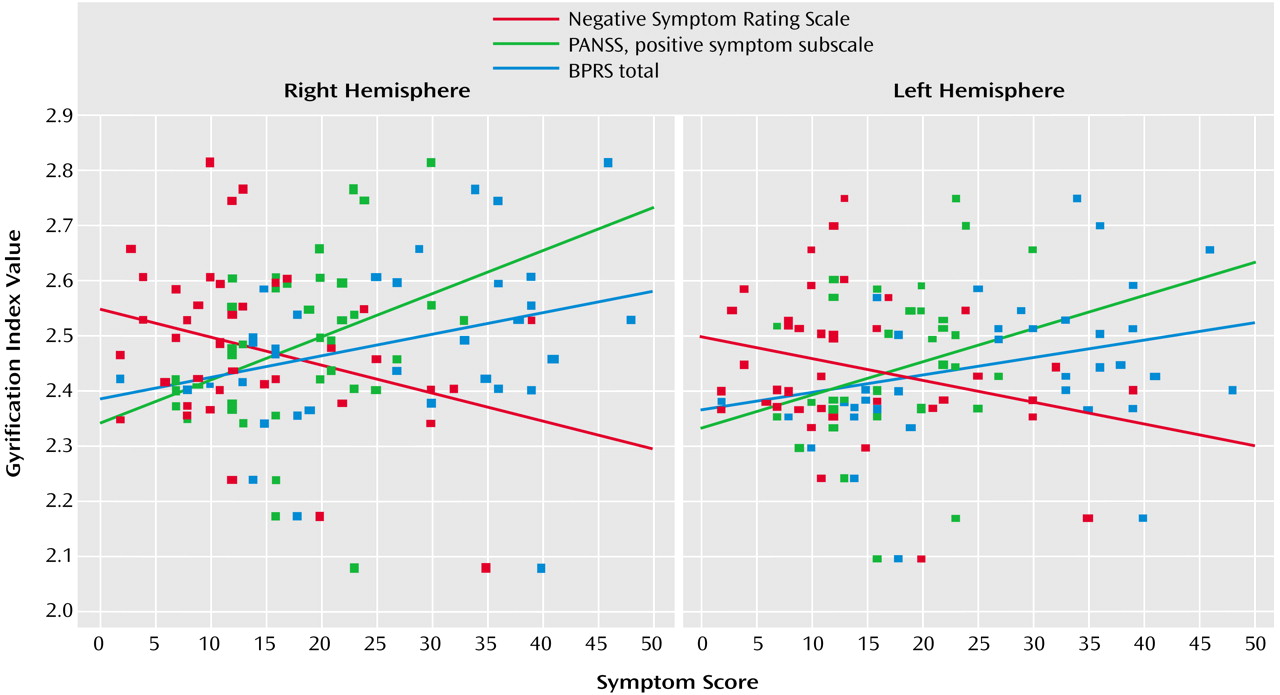
Schizophrenia patients with the paranoid subtype showed similar right gyrification index values to healthy comparison subjects. Indeed, symptom scores that tend to cluster around the paranoid dimension (suspiciousness, delusions, hallucinations, and grandiosity) were associated with an increased gyrification index in our study. On the other hand, the severity of the negative symptoms such as deficits of attention, orientation, and motivation were inversely related to the values of cortical folding. This may be related to the fact that, within the schizophrenic group, the disorganized subtype showed a lower gyrification index, mainly due to reductions in the left hemisphere. However, this interpretation must be made with caution, since the correlation coefficients were relatively modest (rs around –0.35 and p<0.05) and the elevated number of correlations increased the risk of false-positive results.
Several previous neuroimaging studies have found reduced white matter in schizophrenic patients
(12,
29–31). Wible et al.
(29) and Sanfilipo et al.
(31) reported an association between a decreased volume of frontal white matter and the severity of negative symptoms, and Sigmundsson et al.
(12) found reduced white matter in the left hemisphere of patients with prominent negative symptoms. Our study showed that the patients with a high prevalence of deficit symptoms (disorganized subtype) had a lower gyrification index. Supposing that a volumetric reduction in the white matter corresponds to a reduced number of axons, lower viscoelastic tension, and therefore a lower cortical folding, our results are in consonance with the findings reported by those authors.
In summary, the hypothesis that abnormalities in cortical folding represent underlying disturbed neuronal connectivity is based on morphogenetic and neuropathologic studies involving the subplate in the genesis of corticocortical and corticothalamic connections as determinant factors of viscoelastic tension responsible for cortical folding. Therefore, the reduced gyrification index observed in schizophrenic patients may reflect abnormalities in corticogenesis and may be related to negative symptoms (e. g., deficits in attention, orientation, and motivation).
However, gyrification index is only an indirect measure of a neuropathological process that is still poorly understood. Additional empirical investigations are needed in order to clarify how the gyrification index could be affected by neurodevelopmental disturbances in humans and to ascertain what kind of cytoarchitectural abnormalities could be related with disturbances in cortical folding.
Our study had limitations that need to be acknowledged. Not all the subtypes of schizophrenia were represented in the study (e.g., the catatonic subtype), and the relatively small number of subjects made the statistical analysis of subgroups problematic. On the other hand, the symptom-based subtypes of DSM have a very modest stability along the course of illness. Patients initially diagnosed as one subtype may display a combination of symptoms that changes over time to another subtype
(5), thereby making it difficult to establish the homogeneity of subgroups of schizophrenia.
In conclusion, we found a pattern of reduced cortical folding in patients with schizophrenia that may reflect cytoarchitectonic abnormalities in the neurodevelopmental process. This phenomenon was mainly pronounced in patients with the disorganized subtype, with predominance of negative symptoms. On the other hand, the paranoid subtype showed a relatively preserved gyrification index that correlated positively with the severity of positive symptoms.
In addition, schizophrenic patients showed significantly reduced cortical folding in the left hemisphere compared with the right hemisphere. Such a result may indicate a main effect of left lateralization in schizophrenic patients, a finding that is also in line with abnormal neurodevelopment.