Human in vivo imaging studies have documented significant dopamine transporter loss in methamphetamine users
(1,
2). In our previous positron emission tomography (PET) study
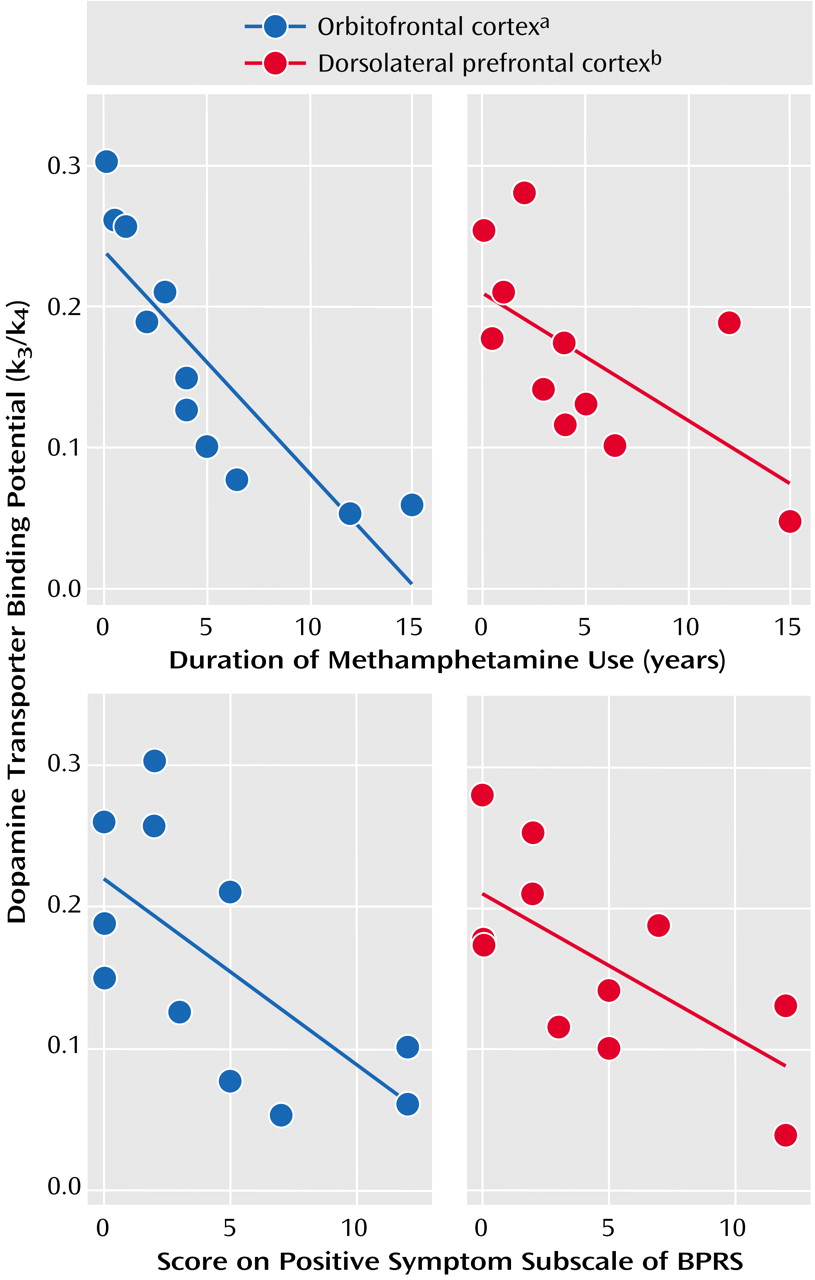
(3), we found significant dopamine transporter reduction in the striatum and nucleus accumbens of methamphetamine users; in addition, this reduction was associated with the duration of methamphetamine use and the severity of positive symptoms. However, our previous study did not address the importance of the orbitofrontal cortex and dorsolateral prefrontal cortex in methamphetamine-associated psychiatric states, although two studies dealing with this issue have been published since the publication of our article
(4,
5). A PET study by Volkow et al.
(4) assessed the availability of dopamine D
2 receptors in methamphetamine users in addition to investigating regional brain glucose metabolism as a marker of brain function. Methamphetamine users had a significantly lower level of D
2 receptors in the striatum than was observed in comparison subjects; this decrease was associated with the dysregulation of orbitofrontal cortex function. A functional magnetic resonance imaging (fMRI) study by Paulus et al.
(5) demonstrated that methamphetamine users failed to exhibit normal activation in both the orbitofrontal cortex and the dorsolateral prefrontal cortex during a decision-making task. Therefore, we reevaluated dopamine transporter density in the orbitofrontal cortex and dorsolateral prefrontal cortex in methamphetamine users in terms of the extent to which this density was associated with clinical characteristics, including psychotic symptoms, duration of methamphetamine use, and craving for methamphetamine. In the current study, we also considered dopamine transporter density in the amygdala, which has dopaminergic connections with both the orbitofrontal cortex and the dorsolateral prefrontal cortex
(6).
Method
This study was approved by the Ethics Committee of the Hamamatsu University School of Medicine. After complete description of the study, written informed consent was obtained from all subjects before study entry.
Eleven male abstinent methamphetamine users (mean age=27.4 years, SD=5.6) and nine healthy male comparison subjects (mean age=26.9 years, SD=4.5) were evaluated in this study. These participants were the same as those in our previous study
(3). The comparison subjects had no history of psychiatric disorder or substance abuse. None of the methamphetamine users had a history of psychiatric symptoms before the use of methamphetamine, and none had used any drugs other than methamphetamine. The demographic characteristics of the methamphetamine users have been described elsewhere in detail
(3). Briefly, the duration of methamphetamine use ranged from 1 month to 15 years (mean=4.8 years, SD=4.8), and methamphetamine abstinence ranged from 7 days to 1.5 years (mean=5.6 months, SD=5.7). At the time of the PET scan, three methamphetamine users had no psychiatric symptoms, four had persistent anxiety disorder-like symptoms, and four had persistent psychotic symptoms as determined by the Brief Psychiatric Rating Scale (BPRS). The Subjective Craving Scale for Alcohol Dependence was slightly modified and used for the assessment of methamphetamine craving.
To evaluate dopamine transporter density in the orbitofrontal cortex, dorsolateral prefrontal cortex, and amygdala, we reanalyzed the PET scan data from our original study
(3). PET scans were performed by using an SHR2400 PET scanner (Hamamatsu Photonics K.K., Hamamatsu, Japan) following intravenous injection of [
11C]WIN 35,428. A sequence of 38 PET scans was obtained over 90 minutes. Arterial blood samples were drawn at intervals of 10 seconds to 15 minutes after the tracer injection. On the basis of a three-compartment and four-parameter (K
1, k
2, k
3, k
4) model, dopamine transporter density, represented by the binding potential (k
3/k
4), was calculated as previously described
(3,
7). The frontal regions studied here, i.e., the orbitofrontal and dorsolateral prefrontal cortices, are known to be sensitive to the partial volume effect of cortical atrophy due to substance use
(8). To minimize the contribution of the partial volume effect, magnetic resonance images were superimposed onto PET images according to previously described procedures
(7). Subsequently, by referring to areas on the magnetic resonance images as anatomical landmarks, regions of interest were carefully drawn to avoid the involvement of the sulci.
In order to compare the values of the binding data between methamphetamine users and healthy comparison subjects, the Mann-Whitney U test was used. Correlations between dopamine transporter binding and the clinical parameters (duration of methamphetamine use, duration of abstinence, BPRS score, and craving score) were evaluated with Kendall’s tau. Statistical significance was set at p<0.05.
Discussion
To our knowledge, this is the first study to report a significant reduction in dopamine transporter density in the orbitofrontal cortex, dorsolateral prefrontal cortex, and amygdala in methamphetamine users. When taken together with the results of our previous study showing a significant decrease in dopamine transporter density for the striatum, nucleus accumbens, and anterior prefrontal cortex
(3), it is suggested that chronic intake of methamphetamine leads to dopamine transporter reduction in discrete brain areas that receive dopaminergic input. The dopamine transporter reduction in the orbitofrontal cortex and the dorsolateral prefrontal cortex was significantly associated with the duration of methamphetamine use and with the severity of persistent psychiatric symptoms. This finding further supports our contention that a longer period of methamphetamine use may cause a relatively greater reduction in dopamine transporter density in the brain, a result that may also be closely associated with the severity of psychiatric symptoms. In this study, no correlation was found between dopamine transporter density and the duration of abstinence (mean=6 months). However, this result does not necessarily indicate that the reduction in dopamine transporters is irreversible, since Volkow et al.
(9) have provided evidence of a significant recovery of dopamine transporter density with protracted abstinence (mean=17 months).
A recent PET study suggested dopamine D
2 receptor-mediated dysregulation in the orbitofrontal cortex of methamphetamine users
(4). Moreover, dysfunction of the orbitofrontal cortex and the dorsolateral prefrontal cortex during decision making was demonstrated by a recent fMRI study
(5). It is possible that the dopamine transporter may be a specific site damaged by methamphetamine, and the dopaminergic system is likely to be actively involved in the function of the orbitofrontal cortex and dorsolateral prefrontal cortex. However, it remains unknown whether or not the observed dopamine transporter reduction participates in the functional disruption of the orbitofrontal cortex and dorsolateral prefrontal cortex. If there were indeed such a direct influence, determination of its mechanism would be of great interest. Hence, further work in this area is still needed.
Unlike in the orbitofrontal cortex and dorsolateral prefrontal cortex, dopamine transporter density in the amygdala was not correlated with any of the clinical markers discussed here. A larger study would be of benefit, as would the use of a tracer with a higher specific-to-nonspecific binding ratio, in order to clarify the differences between dopamine transporter density in the amygdala and that in the other areas of the brain studied here.