Depression and coronary artery disease frequently co-occur in the same individuals. In fact, a number of epidemiological studies have documented that depression is not only a prognostic factor in patients with established coronary artery disease
(1) but also a risk factor for the incidence of cardiovascular disease in previously healthy subjects
(2). Several mechanisms have been suggested to explain the observed links between depression and coronary artery disease, including poor compliance with medication and lifestyle changes, lower heart rate variability, and greater platelet activation
(3–
6). Of more recent interest is the role of inflammation in the development and progression of coronary artery disease
(7) and its potential association with depression
(8).
Inflammation is an intrinsic part of atherosclerosis
(7,
9) in which development of an atherosclerotic plaque is associated with both local and systemic release of cytokines, such as interleukin-6 (IL-6), hepatocyte production of proteins known as acute-phase reactants, such as C-reactive protein, and the expression and endothelial shedding of soluble intercellular adhesion molecules (sICAMs), such as sICAM-1. Since atherosclerosis is a chronic disease, this systemic, low-grade pattern of immune activation, known as the acute-phase response, tends to persist in patients with coronary artery disease and is particularly active at the time of an acute coronary episode. These markers of inflammation also predict the incidence of coronary artery disease
(10–
12) and a worse prognosis following an acute coronary syndrome
(13–
15).
It is intriguing that patients with depression show patterns of immune activation similar to those seen in coronary artery disease
(16,
17). A meta-analysis of studies of depressed patients without coronary artery disease
(18) showed that the most consistent pattern of immune activation involved high circulating levels of IL-6 and soluble IL-2 receptor and high numbers of lymphocytes expressing activation markers (e.g., IL-2 receptor). More recent studies have also shown high levels of acute-phase proteins
(19,
20) and soluble adhesion molecules
(21). However, to our knowledge only one small study (N=30) has looked at the relationship between depression and markers of inflammation in patients with coronary artery disease. Appels et al.
(22) found that major depression and depressive symptoms, measured by a group of questions included in the Vital Exhaustion Interview, were associated with high IL-1β and IL-6 levels 1 hour before angioplasty.
Given the well-documented role of subchronic inflammation in coronary artery disease and its putative relationship with depression, we conducted the current study to examine the relationship between depression and serum markers of inflammation in a large group of patients recently discharged after hospitalization for acute coronary syndromes. More specifically, our hypotheses were that major depression would be associated with high levels of IL-6, sICAM-1, and C-reactive protein in patients recovering from acute coronary syndromes and that the relationships between depression and inflammation would remain independent of potential confounders, that is, factors associated with both depression and markers of inflammation.
Method
Subject Selection
Patients were assessed at the Montreal Heart Institute Research Center. All consecutive patients undergoing a coronary angiogram for a suspected acute myocardial infarction or episode of high-risk unstable angina (confirmed by elevation of troponin T level) at one of two cardiac centers in Montreal between Aug. 31, 1999, and Aug. 2, 2001 (N=2,716), were considered potentially eligible for study participation. The thresholds for determining abnormal troponin T levels were based on each hospital’s laboratory recommendations. A patient was excluded if the acute coronary syndrome was secondary to another medical disease or if he or she had a life-threatening disease likely to limit survival to less than 2 years, was cognitively unable to collaborate or provide informed consent, was unlikely to be able to come to the research center for evaluation because of distance, or had insufficient knowledge of French or English to complete psychosocial interviews (899 exclusions). The patients completed baseline interviews, and blood samples were obtained approximately 2 months after hospital discharge as part of the Epidemiological Study of Acute Coronary Syndromes and the Pathophysiology of Emotions, a study assessing the pathophysiological mechanisms of depression in patients with acute coronary syndrome. This study received approval from the research ethics committees of the Montreal Heart Institute and Hôpital du Sacré-Coeur de Montréal.
Soon after discharge, eligible patients received brief letters describing the study and giving them a telephone number to call if they did not want to receive a telephone call providing more information. Approximately 6 weeks after discharge, repeated attempts were made to telephone all eligible patients to explain the study and obtain preliminary agreement to participate. Calls were made to 1,577 patients, and 965 agreed to take part and were given appointments at the research clinic to complete the informed consent process and take part in the study.
After the patients arrived at the research center following an overnight fast, the study procedures, including blood drawings and interviews, were explained. Informed consent was provided by 812 patients (51.5% of those eligible). The major reasons for lack of study participation were the blood-sampling procedures, including the requirement for an overnight fast, and the long time commitment needed (approximately 3 hours). Following a brief rest period, antecubital blood was drawn to assess lipids, glucose, and insulin. Samples for IL-6 and sICAM-1 were also collected from the first 500 patients recruited from Aug. 31, 1999, through March 7, 2001. Patients taking antibiotics, those with current alcohol abuse, and those not fasting at the time of the blood draw were excluded, resulting in a study group of 481 subjects with measurements of inflammatory markers.
Procedure
The blood samples for measurement of IL-6 and sICAM-1 were collected in citrated tubes and centrifuged immediately at 4°C; the plasma aliquots were frozen at –80°C until analyzed. The samples for measurement of C-reactive protein were collected in plain tubes, and the serum aliquots were frozen at –80°C. Blood was drawn between 8:15 and 10:40 a.m.; 95% of the samples were collected before 10:00 a.m. After the patients had a light breakfast, one of two trained psychologists administered the Structured Clinical Interview for DSM-IV (SCID)
(23), to assess current and past major depression according to DSM-IV criteria.
Data on revascularization procedures following the admission for the index myocardial infarction or unstable angina, cardiac history, and ventricular function were abstracted from hospital charts. The final diagnoses for the index admission were based on systematic review of all charts using research criteria applied by investigators blind to all other data. The diagnosis of Q-wave myocardial infarction required ECG evidence of new Q waves in two consecutive leads. Non-Q-wave myocardial infarction required a peak creatine phosphokinase enzyme level 1.5 times the hospital level indicating normal, or a creatine phosphokinase myocardial band isoenzyme (CPK-MB) value greater than 5% of a simultaneous creatine phosphokinase value greater than normal, before any angioplasty or coronary artery bypass surgery. The upper limit for a creatine phosphokinase elevation occurring after an angioplasty was three times the normal level, and following coronary artery bypass grafting the upper limit was 10 times the hospital’s upper normal limit for CPK-MB mass. All other patients had a research diagnosis of high-risk unstable angina (high level of troponin T).
The number of major coronary arteries with 50% or greater stenosis on the angiogram before revascularization procedures was determined by an experienced cardiac radiologist, blind to psychiatric data and other patient characteristics, using established research criteria
(24).
Presence or absence of the metabolic syndrome and classification of its individual components were based on criteria included in a report by the Adult Treatment Panel III of the National Cholesterol Education Program
(25) and referred to in a recent publication by the Third National Health and Nutrition Examination Survey
(26). The following five components were included: abdominal obesity (waist circumference >102 cm in men and >88 cm in women), hypertriglyceridemia (fasting serum triglycerides >1.69 mmol/liter), low level of high-density lipoprotein (HDL) cholesterol (fasting HDL <1.04 mmol/liter in men and <1.29 mmol/liter in women), high blood pressure (≥130/85 mmHg or use of diuretics), and high fasting glucose level (fasting glucose ≥6.1 mmol/liter or use of antidiabetic medication). While the investigators in the third national Health and Nutrition Examination Survey classified patients currently taking antihypertensive medication as hypertensive, we had no information about the reason for prescription of beta-blockers, angiotensin-converting enzyme (ACE) inhibitors, and calcium channel blockers for our subjects, and only diuretics were included as part of the classification of high blood pressure. Patients with more than two components were classified as having the metabolic syndrome. Current medications other than diuretics were analyzed separately.
Laboratory analysis of inflammatory markers was carried out at the Cousins Center for Psychoneuroimmunology and at the Montreal Heart Institute Research Center. The stored aliquots were thawed and blindly assayed for inflammatory markers by using commercially available ELISA kits. Levels of C-reactive protein were measured by means of the Dade Behring N High Sensitivity assay (Dade Behring Diagnostics, Marburg, Germany) on the BN ProSpec (Dade Behring). Levels of IL-6 and sICAM-1 were measured in duplicate with commercially available ELISA (R&D Systems, Minneapolis); the mean coefficients of variation were 7.2% and 5.1%, respectively.
Statistical Analyses
Analyses were carried out with SPSS for Windows, version 10.0
(27). The data for all inflammatory markers and the fasting insulin levels were skewed, and analyses for these variables were based on natural log transformations. The transformations resulted in nearly normal distributions of the variables, as well as their residuals in subsequent multiple linear regression analyses. Differences between depressed and nondepressed patients on continuous background variables and inflammatory markers were evaluated by using analysis of variance (ANOVA), and differences on categorical variables were analyzed by using chi-square tests. Multiple linear regression analysis was used to assess the relationships between the inflammatory markers and continuous background variables as well as the two-way interactions between depression and the potential moderating variables of age, sex, past depression, and statin use for each inflammatory marker. Variables that were associated (p<0.10) with both major depression and inflammatory markers were selected for covariate control. Multiple linear regression analysis was used to adjust the relationships between major depression and inflammatory markers by entering the covariates in the first step of the regression and then adding depression.
Results
Subject Characteristics
The 481 patients with inflammatory marker data were evaluated between 23 and 120 days after hospital discharge (mean=59.7 days). Their mean age was 60 years (range=26–90), and 18.9% were women. Most (81.7%) had had an acute myocardial infarction (Q-wave myocardial infarction, 22.5%) at the time of the index hospitalization, 19.3% had undergone coronary artery bypass surgery at that time, and 49.1% were classified as having the metabolic syndrome. The DSM-IV criteria for a current major depressive episode were met by 7.3% of the study group, with 4.4% experiencing a first depression. The overall lifetime prevalence of major depression was 21.2%.
Inflammatory Markers and Major Depression
The levels of the three inflammatory markers were significantly related, and the correlation between IL-6 and C-reactive protein (r=0.58, N=481, p<0.001) was somewhat higher than the correlation between sICAM-1 and IL-6 (r=0.17, N=481, p<0.001) or between sICAM-1 and C-reactive protein (r=0.21, N=481, p<0.001).
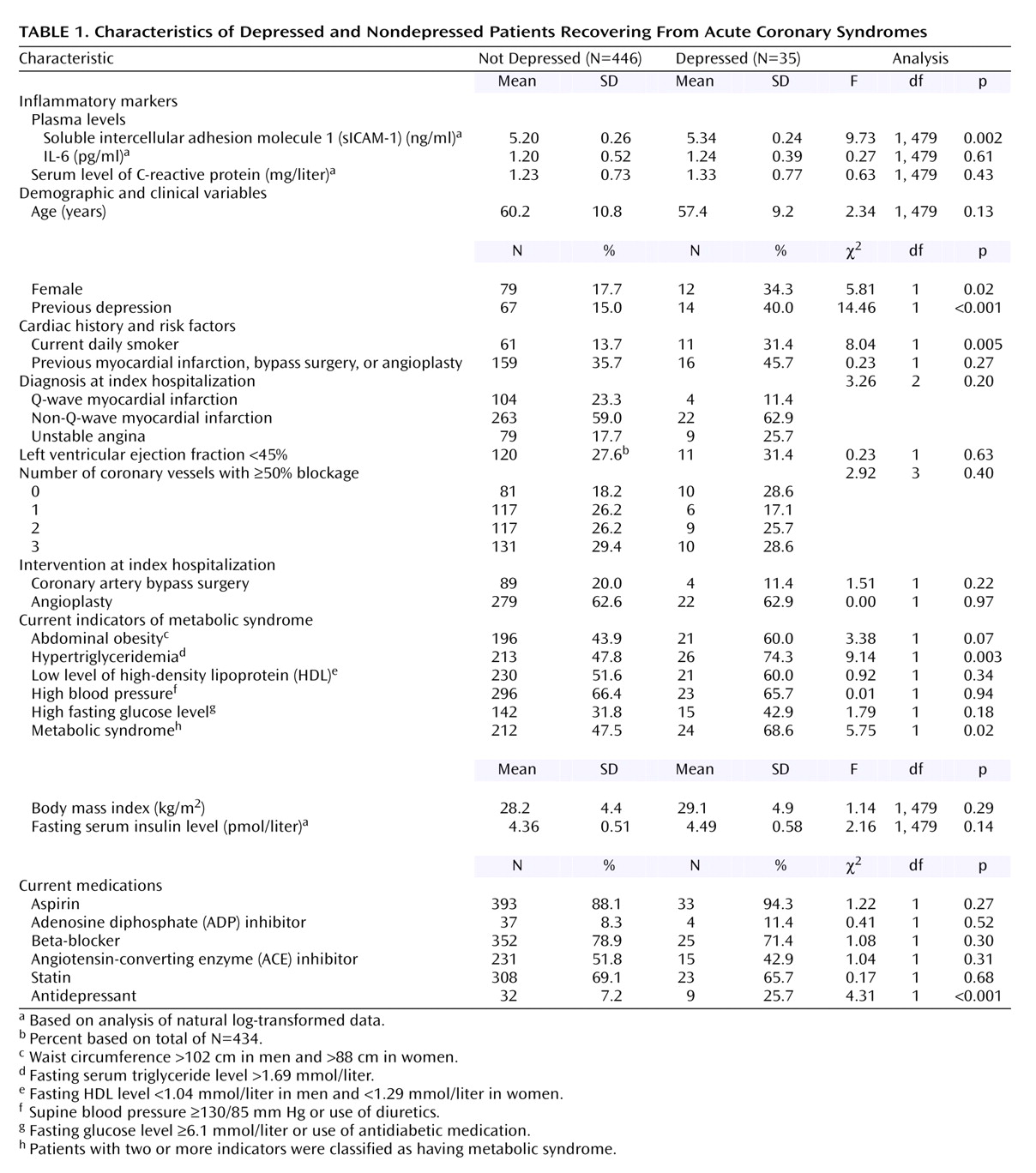
Table 1 shows the mean values of the inflammatory markers and other baseline characteristics for subjects with current major depression and those without.
sICAM-1 was the only inflammatory marker significantly related to current major depression (
Table 1) . There was also a significant interaction between current and past depression (F=3.98, df=1, 477, p=0.05). To illustrate this interaction,
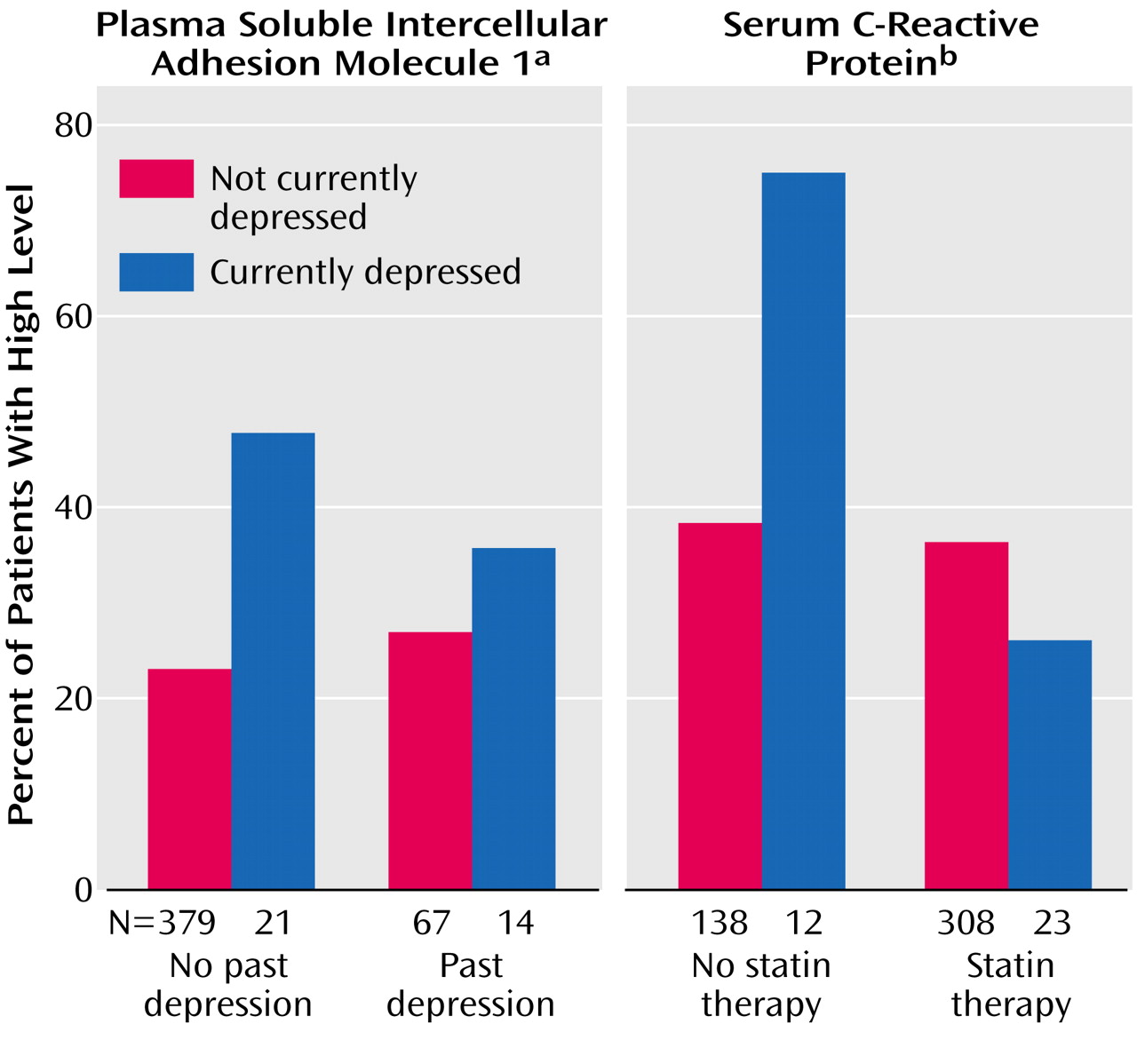
Figure 1 uses the group’s upper quartile of sICAM-1 levels (≥210 ng/ml) since there are no established cutoff values for high sICAM-1 levels. Although the currently depressed patients tended to have higher sICAM-1 levels regardless of whether or not they had a previous depression (main effect from ANOVA: F=9.18, df=1, 477, p=0.003), the link between current depression and sICAM-1 was much more marked in the individuals with a first depression. None of the interactions between age or sex and current depression for the inflammatory markers had a p value less than 0.20.
Besides having higher levels of sICAM-1, the depressed were also more likely to be women, to have had a previous depression, and to smoke daily, as well as to take antidepressants (
Table 1). In addition, there was a significantly higher rate of metabolic syndrome among the depressed, largely due to a higher rate of hypertriglyceridemia and a nonsignificantly higher rate of abdominal obesity. The depressed and nondepressed patients did not differ significantly in body mass index, insulin level, diagnosis at the time of the index admission, or the number of major blockages on the cardiac angiogram.
Adjustment for Covariates
In addition to the diagnosis of major depression, sICAM-1 levels were significantly related to older age (r=0.11, N=481, p=0.02), female sex (F=13.79, df=1, 479, p<0.001), smoking (F=7.03, df=1, 479, p=0.008), previous myocardial infarction or coronary revascularization procedure (F=7.84, df=1, 479, p=0.005), and the metabolic syndrome (F=18.76, df=1, 479, p<0.001), including all of its individual components except hypertension (p=0.15). Body mass index was also significantly related to sICAM-1 (r=0.14, N=481, p=0.002). Patients taking adenosine diphosphate (ADP) inhibitors had significantly higher sICAM-1 levels than other patients (F=5.69, df=1, 479, p=0.02). Those taking antidepressants (F=2.81, df=1, 479, p=0.10) and those not receiving statin therapy (F=3.40, df=1, 479, p=0.07) had marginally higher sICAM-1 levels. The sICAM-1 level was not significantly related to past depression, left ventricular ejection fraction, number of vessels blocked on the coronary angiogram, coronary bypass surgery at index hospitalization, or prescription of aspirin, beta-blockers, or ACE inhibitors (for all, p>0.40).
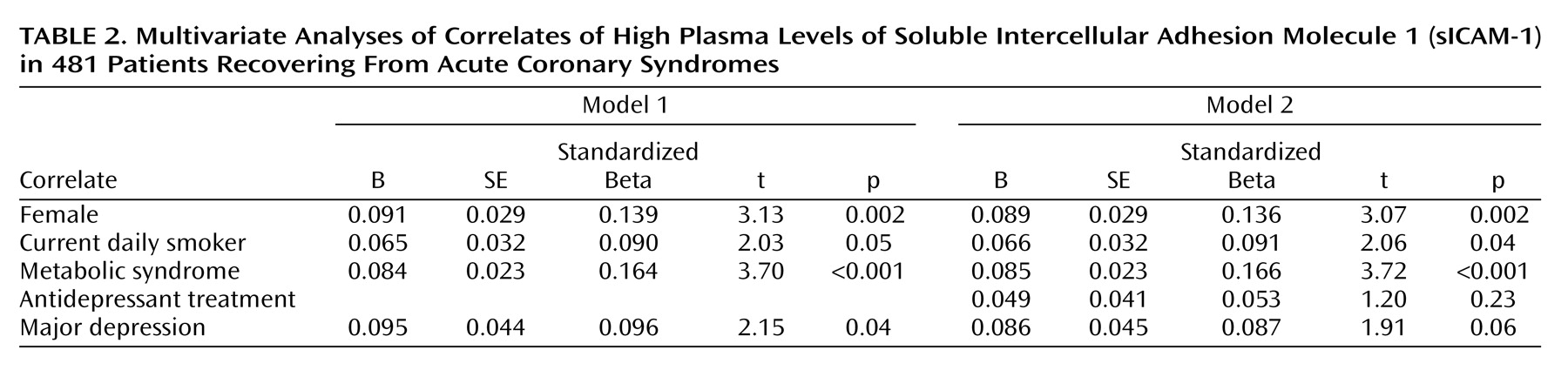
Variables that were at least marginally related to both sICAM-1 level and major depression (p<0.10) were selected as potential confounders of the observed link between the two and entered in the first step of a multiple linear regression analysis to predict sICAM-1 level. Major depression was entered in the second step. As shown in
Table 2, the difference in sICAM-1 levels between patients with major depression and those without remained significant (p=0.04) after statistical control for sex, current smoking, and the presence of the metabolic syndrome, suggesting that the link between depression and sICAM-1 was not explained by these factors. The relationship was slightly attenuated when antidepressant treatment was added to the model (p=0.06). The results were similar when abdominal obesity and hypertriglyceridemia were substituted for the metabolic syndrome classification. The interaction between current and past depression remained at least marginally significant after adjustment for the covariates in all models (for all, p<0.07).
Role of Statins
All patients had documented coronary artery disease, and although they were stable, their cardiac medications could not be safely withdrawn. These medications included statins, which are known to influence inflammatory markers
(28). It is conceivable that statin treatment reduced levels of inflammatory markers in our study group and obscured differences between the depressed and nondepressed groups. In fact, prescription of statins showed a nearly significant association with all three markers. To determine whether statin therapy may have masked the relationship between depression and inflammation, we used ANOVA to assess the two-way interaction between depression and statins for each of the three inflammatory markers. The interaction for C-reactive protein was significant (F=6.35, df=1, 477, p=0.02). This interaction is illustrated in
Figure 1, for which a cutoff value of 3.0 mg/liter was used to determine high levels of C-reactive protein, as suggested by Liuzzo and Biasucci
(9). While depression was associated with higher levels of C-reactive protein in patients not taking statins (for continuous log-transformed data, F=7.10, df=1, 148, p=0.009), there was no relationship in those receiving statin therapy (p=0.41). The interactions for IL-6 (p=0.59) and sICAM-1 (p=0.11) were not significant.
Higher levels of C-reactive protein were significantly associated with age (r=0.10, N=481, p=0.03), female sex (F=5.29, df=1, 479, p=0.03), previous myocardial infarction or coronary revascularization procedure (F=5.97, df=1, 479, p=0.02), the metabolic syndrome (F=11.24, df=1, 479, p=0.001) (including all components except triglyceride and HDL levels), and body mass index (r=0.25, N=481, p<0.001). When the factors significantly related to CRP and to major depression (sex and metabolic syndrome) were entered along with major depression and statin use into the first step of a multiple linear regression analysis, with the interaction entered in the second step, the interaction of C-reactive protein and statin use remained significant (F=6.57, df=1, 475, p=0.01).
Discussion
These data indicate that in patients who have recently experienced acute coronary events, levels of the endothelial activation marker sICAM-1 are significantly higher among the depressed than among the nondepressed. This relationship is particularly strong in those experiencing a first depression. In addition, the relationship between sICAM-1 and depression remained significant in analyses adjusting for other factors related to both depression and sICAM-1, namely, sex, current smoking, and the presence of the metabolic syndrome. These results were only slightly attenuated by adjustment for antidepressant use.
The factors underlying the high level of sICAM-1 in depression patients with acute coronary syndrome are unknown, and with cross-sectional data of the type we collected, we can only speculate about the chain of causation linking them. Depressed patients have high sympathetic tone at rest and in response to acute stress
(3,
29), and it has been documented that sympathetic and beta-adrenergic activation induce expression of cellular adhesion molecules on lymphocytes and monocytes with shedding of L selectin
(30,
31). Thus, depression could lead to increased sICAM-1 levels thru increased sympathetic activity.
Changes in endothelial function may also accompany depression. Rajagopalan et al.
(21) found that untreated young patients with major depression without known traditional cardiovascular risk factors, such as smoking, hypertension, diabetes, or dyslipidemia, had significantly lower brachial arterial response to reactive hyperemia than age- and sex-matched comparison subjects, a sign of endothelial dysfunction. It is interesting that the investigators also found that the depressed patients had higher levels of the soluble adhesion molecules sICAM-1 and E selectin and the chemokine monocyte chemoattractant protein 1 (MCP-1). These findings provide preliminary evidence that depression, in the absence of traditional risk factors known to impede normal endothelial function, is associated with alteration of nitric oxide pathways.
It is also possible that endothelial activation with the release of sICAM-1 reflects an ongoing chronic inflammatory state associated with atherosclerosis of brain arteries, which itself could contribute to depression
(32,
33). A postmortem study of patients with late-life depression showed high levels of ICAM in the dorsolateral prefrontal cortex
(34), supporting the hypothesis that vascular damage in the brain can cause late-life depression. In fact, in our study group, the link between sICAM-1 and depression was more marked for patients with a first depression than for those with a recurrence of depression. We hypothesize that systemic inflammatory processes particularly active around the time of an episode of acute coronary syndrome may facilitate the onset of depression among a subset of subjects.
High levels of IL-6
(35) and C-reactive protein
(19,
20) have been reported in patients with major depression who did not have coronary artery disease. Thus, we expected that IL-6 and C-reactive protein would be related to depression after the acute coronary syndrome episodes. Since many of our subjects were receiving statin therapy when the blood samples were collected, and statins are associated with reductions in C-reactive protein
(28), we wondered whether associations with depression might have been obscured. It is interesting that among the patients not taking statins, depressed patients were about twice as likely to have high levels of C-reactive protein as nondepressed patients, but in those treated with statins depression was unrelated to C-reactive protein. If confirmed in future research, this suggests that the relationship between depression and cardiac events may be attenuated by statin therapy, making this treatment particularly important for depressed patients.
There are limitations to these results. The study included a single blood sample, precluding any inferences about the time course of the inflammatory markers in relationship to the acute coronary event and/or changes in the severity of depressive symptoms. Also, although, to our knowledge, this study is the largest performed so far, we may have had insufficient power to detect small differences, and this may account for the lack of relationship between IL-6 and depression in our study group. In addition, numerous statistical tests were carried out, including the assessment of interactions for potentially important moderators, i.e., sex, age, past depression, and statin treatment, and some of the significant p values may represent falsely positive results, particularly for background variables. Finally, we had a refusal rate of close to 50%, limiting the generalizability of results to the type of patients with acute coronary syndromes who are willing and able to take part in lengthy psychiatric evaluations and blood tests after discharge. As in any preliminary investigations of this type, our results require confirmation by additional research.
Despite these limitations, the data clearly show higher sICAM-1 levels in depressed patients with recent acute ischemic events, a group known to be at higher risk for mortality. They also support other evidence of a role for endothelial function in the link between depression and coronary artery disease
(21). However, we do not know whether the high sICAM-1 levels among the depressed patients in our study group preceded or followed the acute coronary syndrome episodes that led to their inclusion in the study. Future research will need to investigate whether or not sICAM-1 levels could identify a subgroup of depressed patients at particularly high risk for subsequent major cardiovascular events.