At least 10% of the adult population complains of significant insomnia, and the incidence increases with aging. Chronic insomnia is associated with functional impairments, reduced quality of life, higher risk for depression, and increased utilization of health care services
(1–
5). The higher incidence of insomnia with aging is paralleled by an increased use of hypnotic drugs among older adults. The rate of hypnotic use in the community is at least twice as high (14%) among those age 65 years and older, compared to younger age groups
(3). This rate is even higher among older patients attending medical practices, with 26% of women and 6% of men using sleep medications
(6).
The short-term use of hypnotics may be indicated and effective in the treatment of acute insomnia. However, prolonged use of hypnotics, particularly benzodiazepines, is usually not recommended
(7–
9) because of potential adverse effects (e.g., memory impairments), altered sleep physiology (e.g., reduced stages 3 and 4 sleep, increased beta activity), and risks of tolerance and dependence
(10,
11). In older adults, benzodiazepines may increase the risk of falls and hip fractures
(12), motor vehicle accidents
(13), and even mortality
(14). Prescribing guidelines recommend restricting hypnotic use to no more than 2–4 weeks; yet, more than 65% of those prescribed such medications continue using them for more than 1 year, and as many as 30% for more than 5 years
(3,
15,
16). Prolonged users are mostly older adults who report greater sleep dissatisfaction, higher psychological distress, and more chronic medical illnesses
(2,
17).
Discontinuation of hypnotic medications, particularly benzodiazepines, can pose a significant challenge after prolonged use, even when they have been used at low therapeutic doses
(18,
19). Several physiological (withdrawal symptoms) and psychological factors (anticipatory anxiety, fear of rebound insomnia) can perpetuate the vicious cycle and lead to hypnotic-dependent insomnia
(20–
22). Behavioral therapies have been found to be effective for the management of insomnia in nonmedicated patients
(23–
26). However, only a few studies have evaluated use of behavioral interventions to reduce hypnotic medications among chronic users
(27–
32). The evidence indicates that simply encouraging patients to reduce medications has little effect; rather, setting up a medication taper schedule with weekly visits to assess progress is necessary to achieve significant reductions of hypnotic use. In addition, while such a supervised medication tapering regimen is essential in the initial discontinuation phase, it may not be sufficient to keep patients from taking these medications in the long term because of persistent sleep disturbances. The addition of behavioral treatment specifically targeting insomnia symptoms may attenuate withdrawal symptoms (e.g., rebound insomnia) and prevent relapse
(27). Studies conducted with patients using benzodiazepine medication for anxiety have indicated that a gradual reduction is superior to abrupt discontinuation, that medication tapering is as effective with older as with younger patients, and that the addition of psychological treatment reduces withdrawal symptoms
(33–
37).
Although benzodiazepine discontinuation studies have yielded promising short-term results, relapse rates often exceed 50% at follow-up
(28,
29,
36,
38). In addition, few studies have been conducted with older adults, clearly the segment of the population most at risk for benzodiazepine dependency. Finally, previous studies conducted with patients using benzodiazepine medication for sleep have not used polysomnographic assessment to document changes in sleep patterns during and after benzodiazepine discontinuation.
The objectives of this study were to evaluate the efficacy of three interventions (supervised medication taper, cognitive behavior therapy, and a combination of those two approaches) for benzodiazepine discontinuation in older adults and to examine their short- and long-term effects on subjective and objective sleep patterns, as recorded by patients’ sleep diaries and EEG, respectively. It was expected that a combined approach would be more effective than either supervised medication taper or cognitive behavior therapy alone and that benzodiazepine discontinuation might impair sleep in the short term but would actually improve sleep in the long term.
Method
Patients
Prospective patients who were chronic users of benzodiazepine medication for insomnia and who wished to discontinue these medications were recruited through newspaper advertisements and physicians’ referrals. Inclusion criteria were 1) age 55 years or older, 2) history of using benzodiazepine medication for sleep on more than 50% of nights for at least 3 months, 3) subjective complaints of difficulties initiating and/or maintaining sleep for a minimum of 3 nights per week and for at least 6 months, and 4) presence of marked distress or impaired daytime functioning (fatigue, impaired attention and/or concentration). Because hypnotic medications may mask an underlying insomnia problem, subjects were required to meet criteria 3 and 4 either currently (i.e., while taking medication) or after previous attempts to discontinue the medication. Exclusion criteria were 1) evidence that insomnia was directly related to a medical or psychiatric disorder, 2) presence of sleep apnea (apnea-hypopnea index > 15) or periodic limb movements during sleep (periodic limb movement index with arousal >15), 3) current participation in psychotherapy, 4) use of psychotropic drugs other than benzodiazepines for sleep, 5) presence of major depression or other severe psychopathology (e.g., bipolar disorder, psychosis, alcohol/substance abuse) based on the Structured Clinical Interview for DSM-IV, and 6) cognitive impairment as suggested by a score of less than 23 on the Mini-Mental State Examination
(39). These criteria are consistent with those for primary insomnia and hypnotic-dependent insomnia
(20,
40).
Prospective patients underwent a multistep screening evaluation. Of those who completed an initial telephone screening, 156 were considered eligible for the study and 126 went through a second evaluation consisting of a sleep history interview, a psychological assessment, and a medical history with physical examination. Forty-two persons were excluded after these evaluations because of a psychiatric (N=11), medical (N=6), or other sleep disorder (i.e., sleep apnea) (N=3); because of lack of interest (N=15); or because they did not meet the criteria for duration or frequency of benzodiazepine use (N=7). Of the 84 individuals who underwent the next assessment phase of polysomnography, eight additional subjects were excluded because of sleep apnea or periodic limb movements during sleep.
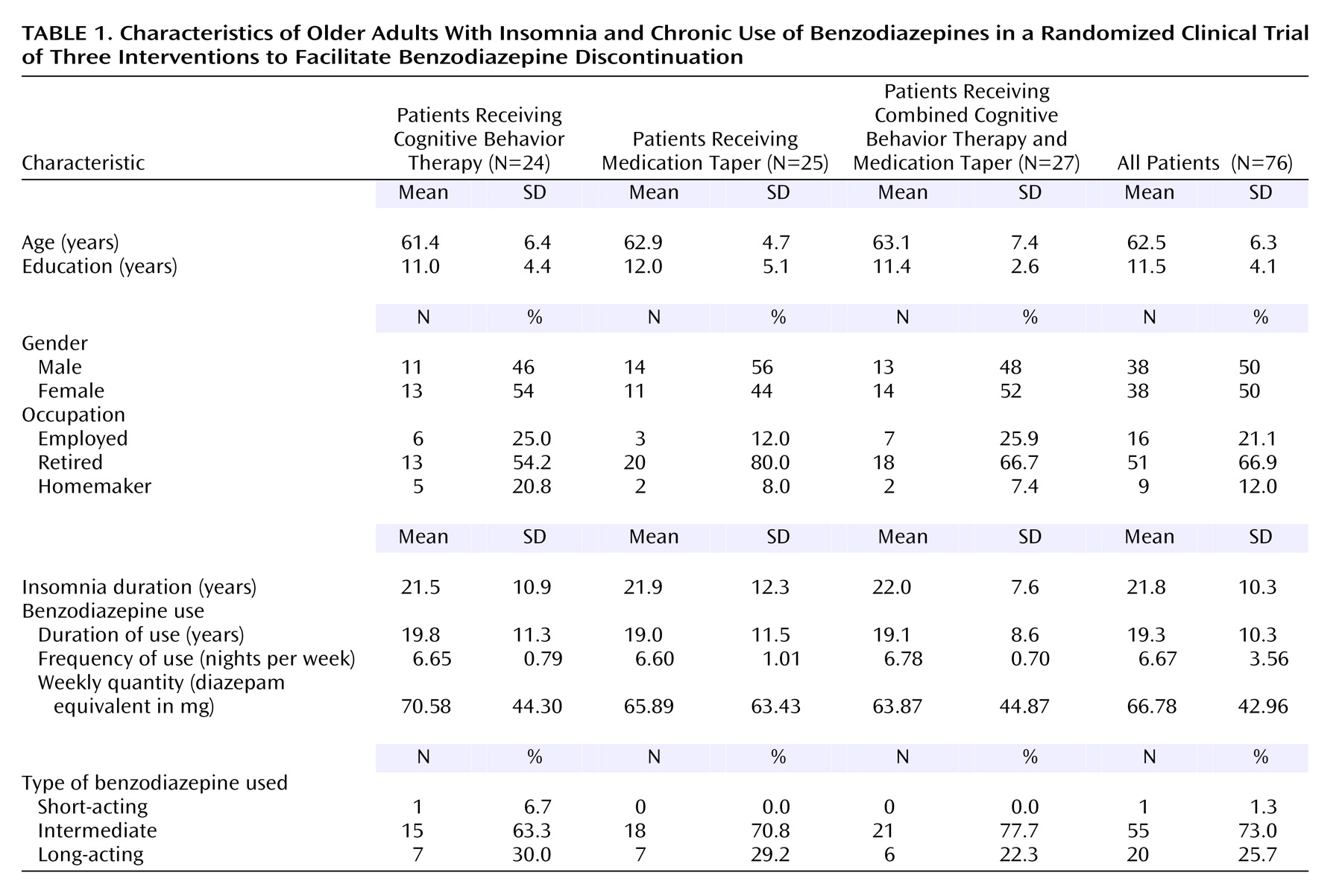
The remaining 76 patients were randomly assigned to one of three treatment conditions: 1) cognitive behavior therapy (N=24), 2) medication taper (N=25), or 3) cognitive behavior therapy plus medication taper (N=27). There were 38 men and 38 women, and the average age was 62.5 years (range=55–82). All patients were white, community-dwelling residents; most were married (75%) and retired (64.5%). While they were taking medication, 64 patients met all of the diagnostic criteria for insomnia and 12 did not meet all the criteria. The majority of the patients (64%) reported mixed sleep-onset and sleep-maintenance insomnia. The average duration of insomnia was 21.8 years. The patients had used benzodiazepine medication for sleep on a regular basis (mean of 6.7 nights/week) for an average duration of 19.3 years (range=2.5–35). The average nightly use was 9.52 mg (diazepam equivalents). Patients had made an average of 6.1 attempts (SD=7.7) to discontinue medications in the past. Most patients (73.0%) used an intermediate-acting benzodiazepine (41.0% used lorazepam, 12.8% used temazepam, 11.5% used bromazepam, 5.1% used oxazepam, and 2.6% used alprazolam), 25.7% used a long-acting agent (flurazepam, clonazepam), and only one patient used a short-acting benzodiazepine (triazolam). Nine patients used two benzodiazepines, three patients used three, and one patient used four.
The study was conducted in a research-based sleep clinic that was part of a teaching hospital. The protocol was approved by the Université Laval Ethics Committee, and all patients provided written informed consent before study entry (i.e., at the initial clinical evaluation).
Table 1 presents the patients’ demographic and clinical characteristics.
Measures
Sleep diaries
Participants kept daily sleep diaries for at least 2 weeks before treatment, during the 10-week treatment period, and for 2 weeks at each of the follow-up assessments. They completed their diaries every morning at breakfast time and brought them in at each consultation session; diaries were mailed in at follow-ups. The diaries were used to monitor several parameters, including bedtime, arising time, sleep-onset latency, number and duration of awakenings, and medication intake. The main outcome variables were benzodiazepine use (frequency and quantity), total wake time (summation of sleep-onset latency, wake time after sleep onset, and early morning awakening), total sleep time, and sleep efficiency (ratio of total sleep time to the actual time spent in bed multiplied by 100). Although sleep diary data do not reflect absolute values obtained from EEG, they provide a reliable index of insomnia
(41) and represent standard outcome assessment in insomnia research
(42). In addition to allowing for prospective monitoring of sleep in the patient’s home environment, sleep diary data reflect an important dimension of insomnia, i.e., the subjective perception of sleep.
Polysomnography
Patients underwent 3 consecutive nights of sleep laboratory evaluation before treatment (within 2 weeks) and at the end of treatment, as well as at 12-month follow-up, for a total of 9 nights of assessment. Bedtime and arising time in the laboratory were kept within one-half hour of the patient’s typical sleep schedule at home. The polysomnography montage included standard EEG, electromyographic (EMG), and electro-oculographic monitoring. Sleep stages were scored according to standard criteria
(43) by an experienced technician who was blind to the patient’s treatment condition. Respiration (airflow, tidal volume, and oxygen saturation) and anterior tibialis EMG were recorded during the first night to detect apnea or periodic limb movements. The dependent variables were the same as those from the diaries (total wake time, total sleep time, and sleep efficiency), plus additional variables related to percentages of time spent in sleep stages 1, 2, 3, and 4 and in REM sleep. Measures of dependent variables were averaged from nights 2 and 3 (baseline), nights 5 and 6 (posttreatment), and nights 8 and 9 (12-month follow-up). To allow for patients’ adaptation to the laboratory, data from the first night of recording at each assessment phase were not used in computing the average measures. Subjects were free from all psychotropic medications, other than benzodiazepine medication, for at least 2 weeks before the baseline evaluations. Subjects were asked to maintain a stable dose of their benzodiazepine medication during this period.
Clinical Outcome Ratings
The Insomnia Severity Index is a 7-item scale that yields a quantitative index of insomnia severity; it was used as a collateral measure of treatment outcome
(22). Ratings on a 0–4-point scale were obtained on the perceived severity of sleep onset, sleep maintenance, and early morning awakening problems; interference with daytime functioning; noticeability of impairment caused by the sleep problem; concern caused by the sleep problem; and satisfaction with current sleep pattern. A composite score is obtained by summing the seven ratings. Higher scores indicate more severe insomnia (total score ranges from 0 to 28). Several items were added to this scale at posttreatment and follow-ups to assess overall degree of improvement, treatment compliance, and satisfaction with the treatment received. A parallel version of the Insomnia Severity Index was also completed by significant others (e.g., spouse). The Insomnia Severity Index has adequate psychometric properties and has been shown to be sensitive to changes in clinical trials of insomnia
(25,
44,
45). The Beck Depression Inventory
(46) and the Beck Anxiety Inventory
(47) were also administered at each assessment phase to monitor anxiety and depressive symptoms.
Withdrawal Symptoms
The patients in the medication taper condition and those in the cognitive behavior therapy plus medication taper condition were evaluated at each treatment visit for withdrawal symptoms. The Clinical Institute Withdrawal Assessment—Benzodiazepines
(48) contains 20 5-point items designed to assess and monitor the type and severity of benzodiazepine-like withdrawal symptoms. The first three items (restlessness, tremor, and sweating) are rated by the treating physician, and the last 17 items are rated by the patient. The total score is the sum of items 1–20. This scale was not used with the patients who received cognitive behavior therapy alone because those patients were not expected to change their medication use and were not provided guidance about reducing their medication intake.
Compliance Measures
Blood and urine samples were obtained on the first night of each series of sleep laboratory evaluation sessions (baseline, posttreatment, and 12-month follow-up). Plasma and urine specimens were tested for evidence of use of benzodiazepines, other sedative-hypnotics, and alcohol. These measures were taken to ensure that potential participants met the study criteria with regard to medication/alcohol use at baseline and to provide validity checks on patients’ reports of their drug intake. Participants were also asked to use the sleep diary to record their adherence to the benzodiazepine taper schedule and to the behavioral homework assignments. Independent ratings of adherence to treatment were obtained from the subjects’ significant others at the posttreatment assessment.
Treatment Conditions
After completing the baseline assessment, the patients were randomly assigned to one of three conditions: supervised benzodiazepine taper, cognitive behavior therapy for insomnia, or a combination of the two interventions. All three treatments were manualized and administered over 10 weekly outpatient consultations.
Supervised medication taper
The patients (N=25) assigned to this condition were provided with a step-by-step withdrawal schedule, with the goal of eliminating benzodiazepine use by the 10th week of treatment. Withdrawal schedules were individualized according to the type of benzodiazepine (short- versus long-acting), dose, and frequency of use. The following principles were used in designing the schedules: 1) setting goals, 2) stabilization with use of a single benzodiazepine for patients who had been using more than one benzodiazepine, 3) reduction of about 25% of the initial dose every 2 weeks until the lowest available dose of the benzodiazepine was reached, 4) introduction of an increasing number of drug-free nights, and 5) scheduled hypnotic use rather than use on an as-needed basis. The specific dose reductions varied as a function of patients’ readiness to discontinue medication and the presence or absence of withdrawal symptoms. However, the time-limited nature of this program was emphasized by setting anchor points. For example, the initial plan was to decrease medication by 25% at week 2, by 50% at mid-treatment, and by 100% at week 10
(19).
Patients enrolled in this condition, either alone or in combination with cognitive behavior therapy, met weekly with a physician for brief consultation sessions (15–20 minutes). The content of those sessions focused on 1) reviewing the written medication taper schedule, 2) documenting changes in insomnia symptoms, and 3) monitoring withdrawal effects and any other adverse events. Support and encouragement to follow the withdrawal schedule were provided, but no specific behavioral recommendations for improving sleep were given during those sessions. Even for patients enrolled in the combined condition, discussion of behavioral sleep management strategies was restricted to cognitive behavior therapy sessions to minimize overlap across conditions.
Cognitive behavior therapy
The patients (N=24) assigned to receive cognitive behavior therapy attended weekly, 90-minute therapy sessions conducted in small groups of four to six patients and led by a master’s-level clinical psychologist. Treatment consisted of a structured, multifaceted, intervention involving behavioral, cognitive, and educational components that targeted different facets of insomnia
(22). The behavioral component incorporated sleep restriction therapy
(49) and stimulus control procedures
(50). Sleep restriction consists of curtailing time in bed to the actual sleep time. For example, if an individual reported sleeping an average of 6 hours per night, of 8 hours spent in bed, then the initial sleep window prescribed for the first week of treatment was 6 hours. This “sleep window” was gradually altered according to the subject’s sleep efficiency (ratio of total sleep time to time in bed) based on the sleep diary data from the previous week. Allowable time in bed was increased by 15–20 minutes when sleep efficiency exceeded 85%, decreased by the same amount of time when sleep efficiency was lower than 80%, and kept stable when sleep efficiency fell between 80% and 85%. Implementation of these rules was flexible, and adjustments were made on the basis of patients’ acceptance and willingness to adhere to the regimen. The allotted time in bed was never less than 5 hours per night. The stimulus control procedures were designed to regulate the sleep-wake schedule and to help patients reassociate the bed/bedroom and bedtime stimuli with sleep rather than with the frustration and anxiety associated with lying in bed trying to sleep. These procedures are 1) going to bed only when sleepy, 2) using the bed and bedroom only for sleep and sex (i.e., no reading, television watching, or worrying in bed or the bedroom either during the daytime or at night), 3) getting out of bed and going to another room if unable to fall asleep within 15–20 minutes, 4) arising at the same time every morning regardless of the amount of sleep obtained the previous night, and 5) avoiding napping (this requirement was optional during the initial sleep restriction phase, except that napping during this phase was limited to less than 1 hour in duration and had to be scheduled before 3:00 p.m.).
The cognitive therapy component was designed to alter faulty beliefs and attitudes that often serve to exacerbate insomnia
(22). Examples of faulty beliefs included 1) unrealistic expectations about sleep requirements (e.g., the absolute need to sleep 8 hours every night), 2) amplifications of the consequences of insomnia (e.g., all daytime impairments are due to poor sleep), 3) erroneous beliefs about strategies to promote sleep (e.g., spending excessive time in bed), and 4) apprehension and misattribution of withdrawal symptoms. In addition, there was an educational component about sleep and aging that was aimed at distinguishing normative from pathological sleep changes occurring in late life and at reviewing sleep hygiene principles about the effects of exercise, caffeine, alcohol, and environmental factors on sleep.
Combined cognitive behavior therapy and taper
The patients (N=27) in the combined cognitive behavior therapy and medication taper condition received both the medication tapering program and cognitive behavior therapy. They attended 10 weekly therapy sessions with a physician to discuss medication management issues and 10 weekly therapy sessions with a psychologist to review behavioral procedures.
Therapists
Cognitive behavior therapy sessions were led by master’s-level licensed clinical psychologists. Before participating in this study, the therapists had previously treated a minimum of four patients by using this treatment. A detailed manual outlining each session was utilized
(22). Two physicians (a general practitioner and a psychiatrist) provided consultations for the medication tapering condition. A detailed manual was also used for the medication tapering sessions. This manual outlined the structure of each consultation session, issues that needed to be covered, and information that was not allowed to be discussed (i.e., behavioral recommendations). All therapy sessions were audiotaped and reviewed regularly with the project director to optimize adherence to the treatment protocol.
Follow-Ups
All patients were contacted by mail 3 and 12 months after completing treatment. At each follow-up, they were asked to complete the same questionnaires and sleep diaries (for 2 weeks) administered at baseline and posttreatment. The participants were also asked to complete an additional series of 3 nights of polysomnographic evaluation at the 12-month follow-up.
Data Analysis
The main dependent variables were benzodiazepine use (quantity, frequency, and drug-free status) (
Table 2), selected sleep parameters based on daily diaries and polysomnography (
Table 3), and clinical outcome ratings and psychological symptoms. All data were carefully inspected to identify missing data and outliers and to assess normality
(51). Descriptive and inferential statistics were completed by using SAS 8.2 statistical software
(52). The alpha level was fixed at 5% (two-tailed) for all inferential tests. The analyses were based on a split-plot group-by-time randomized design (three treatments [group] and four assessments—pretreatment, posttreatment, 3-month, and 12-month follow-up [time]). Data were analyzed within an intent-to-treat framework. However, no missing data imputation was implemented (e.g., last observation carried forward). Instead, linear mixed models were used to test group, time, and interaction effects for all continuous dependent variables. Satterthwaite F tests were computed because they are typically more robust in the context of nonnormality, unbalanced data, and violations of multisample sphericity
(53). A priori contrasts were used to investigate specific hypotheses, such as pretreatment-posttreatment differences, maintenance of treatment gains, and simple main effects (after a significant group-by-time interaction). A generalized estimating model (SAS CATMOD procedure) was used to estimate the three main effects for the proportion of drug-free subjects.
Results
Of the 76 patients enrolled in the study, 69 completed the 10-week treatment protocol, three completed more than half of the protocol, and four dropped out or were withdrawn before reaching mid-treatment. Of the seven patients (five women and two men) who did not complete treatment, three (two in the medication taper condition and one in the combined cognitive behavior therapy and medication taper condition) discontinued participation due to lack of efficacy or withdrawal symptoms, one (in the cognitive behavior therapy condition) reported that there were too many treatment visits, and three (one in each condition) began another psychotropic medication during the course of the intervention. Statistical analyses are based on data for all 76 subjects who were randomly assigned to a treatment group. There were no significant baseline differences across groups in demographic characteristics; severity and duration of insomnia; quantity, frequency, and duration of benzodiazepine use; number of physical illnesses; and number of medications used.
Use of Benzodiazepine Medication
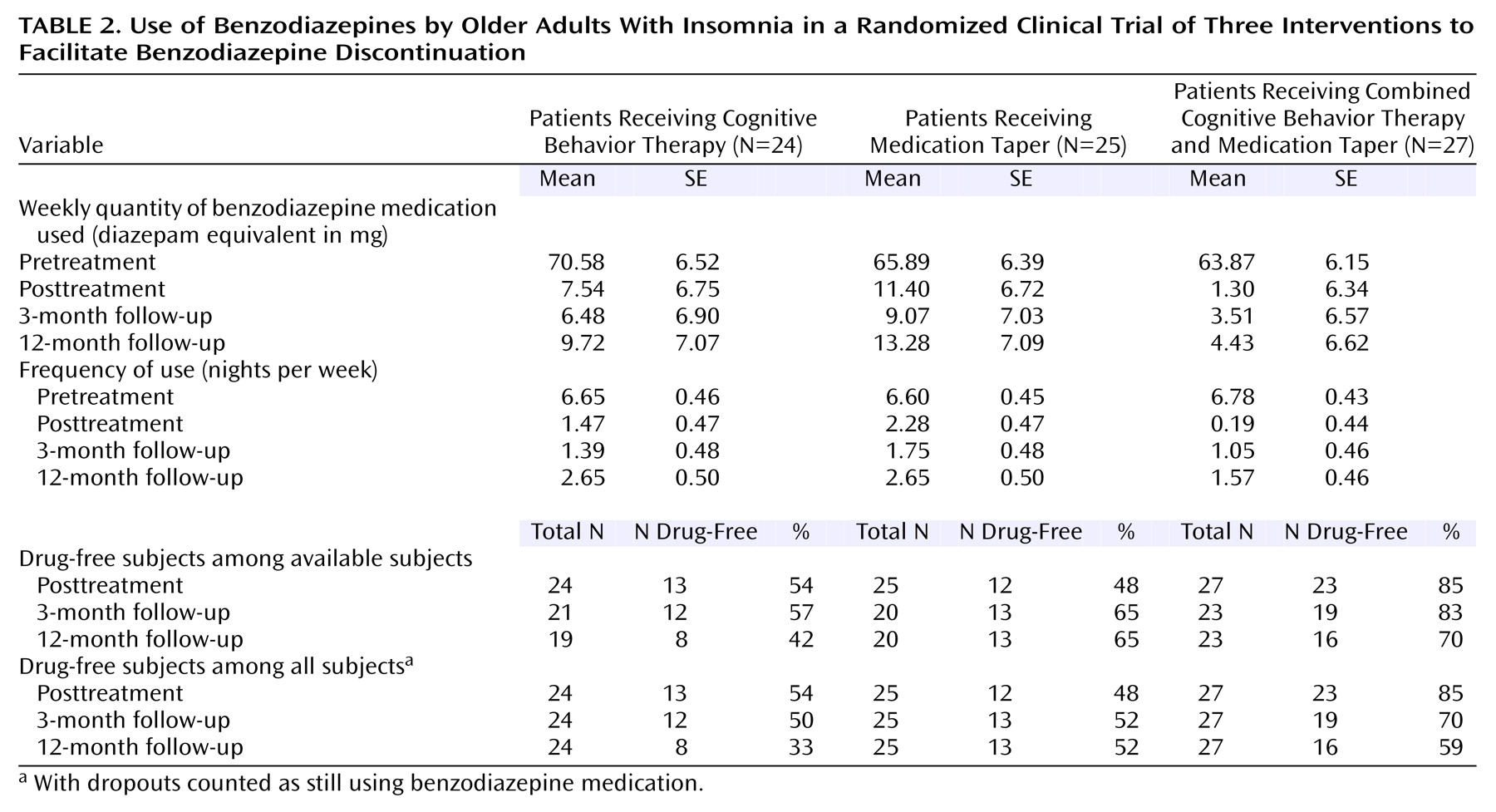
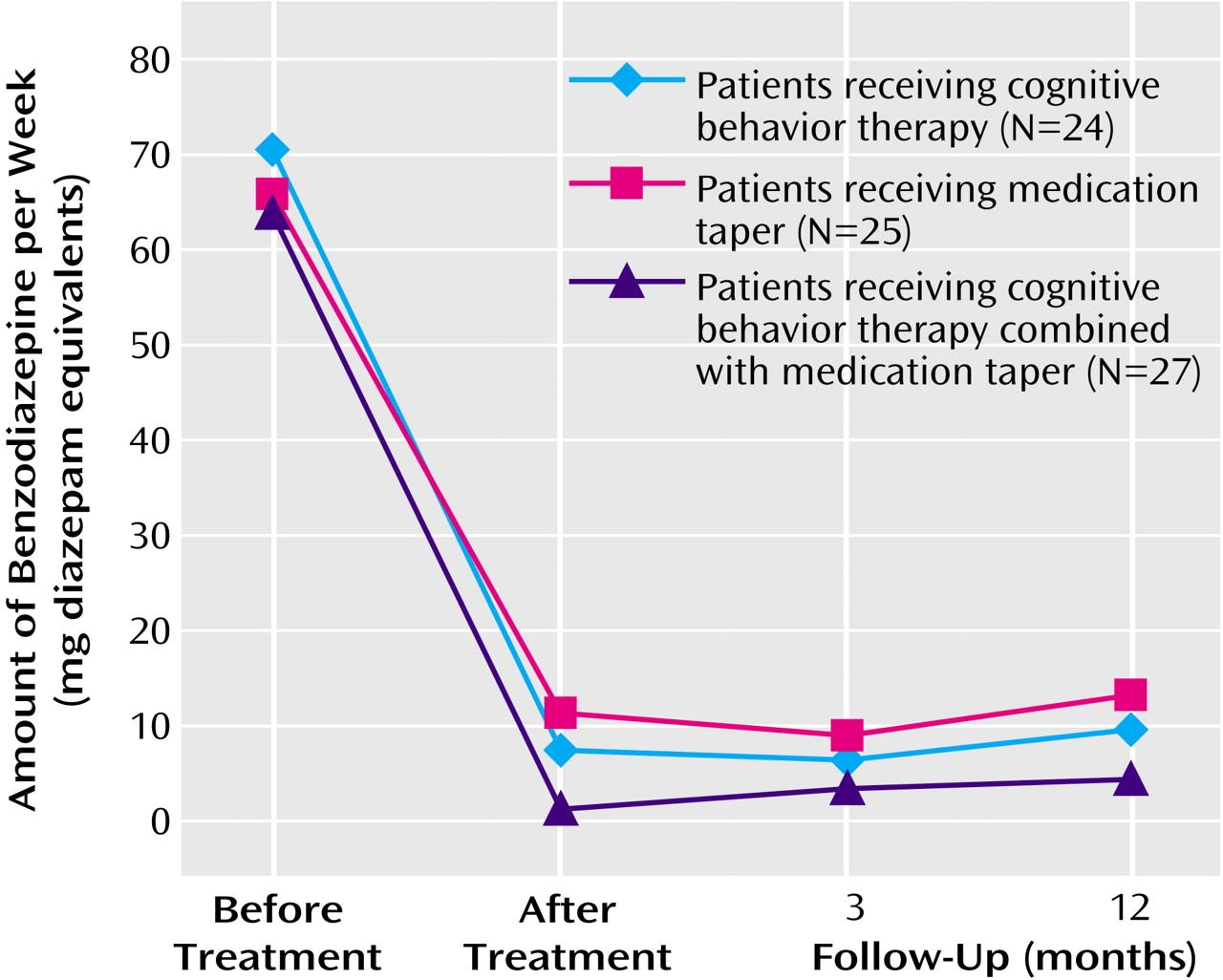
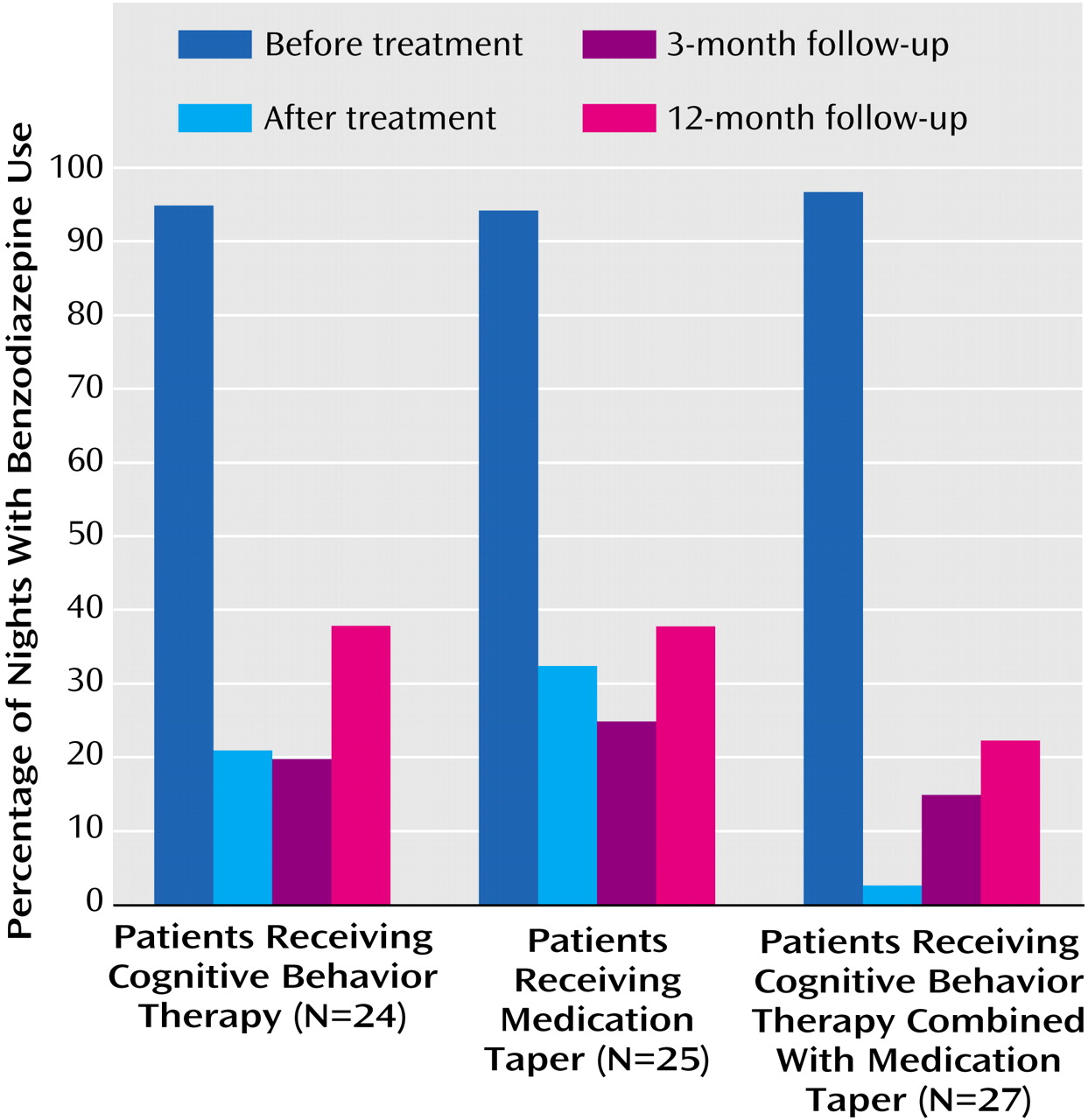
Three variables regarding the use of benzodiazepine medication were analyzed: the average nightly dose of medication used (in diazepam equivalents), the frequency/percentage of medicated nights, and the proportion of patients who were drug-free (i.e., no benzodiazepine used for 2 weeks preceding assessment) (
Table 2,
Figure 1, and
Figure 2). The average nightly dose of benzodiazepine (in diazepam equivalents) decreased from 9.54 mg at baseline to 0.96 mg at posttreatment and 0.91 mg at 3-month follow-up and then increased to 1.31 mg at 12-month follow-up. Linear mixed models showed no significant group effect or group-by-time interaction, but there was a significant time effect (F=88.66, df=3, 126, p=0.0001). A priori contrasts revealed a significant pretreatment-posttreatment effect (F=237.87, df=1, 122, p=0.0001), with an overall reduction of 89.9% in the quantity of benzodiazepine used from baseline to posttreatment. All three groups achieved significant reductions in the quantity of benzodiazepine used during this period (all p<0.0001). There was no other change for any of the treatment groups in the nightly dose of medication used from posttreatment to the 3- and 12-month follow-up assessments.
Combined data for the three groups showed that benzodiazepine medication was used on less than 2 nights per week (1.3 nights, or 18.7% of nights per week) at posttreatment, 1.39 nights (19.9%) at 3-month follow-up, and 2.29 nights (32.7%) at 12-month follow-up, compared to 6.7 nights per week (95.4%) at baseline. There were significant effects for time (F=141.40, df=3, 188, p=0.0001) and the group-by-time interaction (F=2.53, df=6, 187, p=0.02). A priori contrasts revealed significant pretreatment-posttreatment effects for time (F=395.84, df=1, 184, p=0.0001) and the group-by-time interaction (F=6.20, df=2, 184, p=0.003). All three groups had significant decreases in the frequency of medicated nights from baseline to posttreatment (all p<0.0001), with an overall reduction of 80.4% for the three groups combined. In addition, the frequency of medicated nights at posttreatment was lower in the combined cognitive behavior therapy and medication taper group (0.19 night/week) than in the group that received medication taper alone (2.3 nights/week) (F=10.49, df=1, 204, p=0.001). Comparisons of posttreatment data with 3- and 12-month follow-up data showed a significant time effect for medication use frequency (F=5.55, df=2, 187, p=0.005). Overall, there was an increase in the frequency of medicated nights at 12-month follow-up relative to posttreatment.
Sixty-three percent (48 of 76) of the patients were drug-free at posttreatment. A chi-square analysis showed a significant group difference on this variable at posttreatment (χ
2=12.15, df=2, p=0.002). The proportion of drug-free patients was significantly higher in the combined cognitive behavior therapy and medication taper group (85.2%) than in the medication taper group (48%) and the cognitive behavior therapy group (54.2%). Patients took an average of 6.94 weeks (SD=1.73) to discontinue taking benzodiazepine medication, and there was no significant difference between conditions on this variable.
Table 2 shows that the overall proportion of drug-free patients remained near or above 60% at the 3-month (68.8%) and 12-month (59.7%) follow-ups. If we assume that subjects lost to follow-up (i.e., dropouts) were still using benzodiazepine medication, those percentages dropped to 57.9% and 48.7%. The generalized estimating model for the imputed data revealed a significant group effect (χ
2=7.11, df=2, N=76, p<0.03) and a time effect that approached significance (χ
2=5.90, df=2, N=76, p=0.052) but no significant interaction (χ
2=5.48, df=4, N=76, p=0.24). More patients in the combined cognitive behavior therapy and medication taper group remained drug-free at each follow-up, compared with the other groups, even though the between-group differences were no longer statistically significant.
Sleep Data
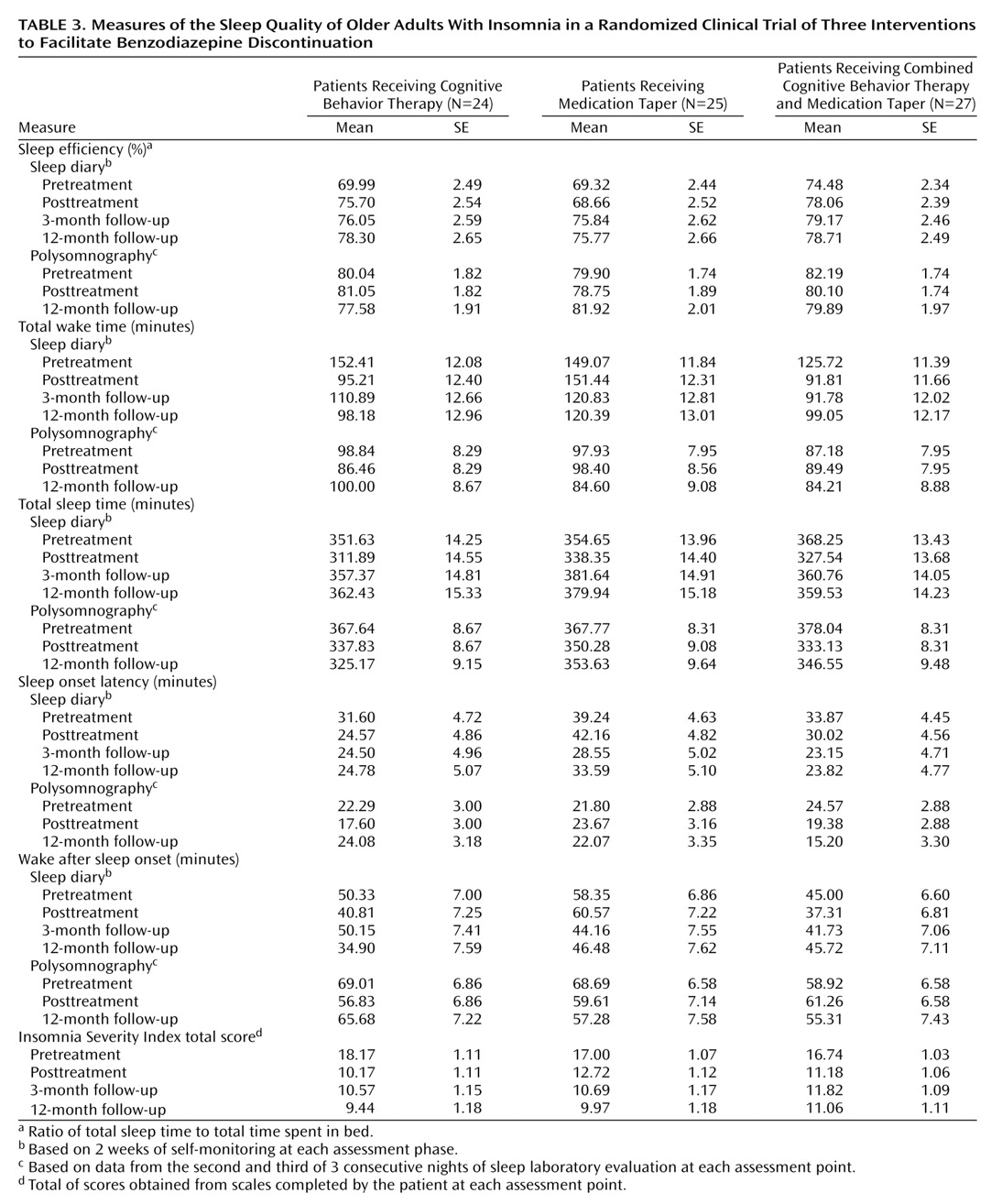
Means and standard errors for selected sleep variables are presented in
Table 3. Sleep diary data are based on 2 weeks of self-monitoring at each assessment phase. Polysomnographic data are based on assessments during nights 2 and 3 at pretreatment, nights 5 and 6 at posttreatment, and nights 8 and 9 at the 12-month follow-up.
Sleep diary
Linear mixed models were computed for five variables derived from the sleep diary. The primary dependent variables were sleep efficiency, total wake time, and total sleep time. The secondary variables were sleep onset latency and wake time after sleep onset. Significant time effects were obtained for sleep efficiency (F=5.60, df=3, 183, p=0.001), total wake time (F=9.63, df=3, 175, p=0.0001), total sleep time (F=20.19, df=3, 185, p=0.0001), and sleep onset latency (F=3.41, df=3, 183, p=0.02). Significant group (F=3.15, df=2, 70, p=0.05) and group-by-time effects (F=3.83, df=6, 175, p=0.0001) were observed for total wake time. A priori contrasts revealed significant pretreatment-posttreatment increases for sleep efficiency (F=6.49, df=1, 183, p=0.01), decreases for total sleep time (F=27.40, df=1, 186, p=0.0001), and decreases for total wake time (F=23.96, df=1, 174, p=0.0001). A significant group effect was obtained at posttreatment for total wake time (F=7.55, df=2, 153, p=0.0007), with all three groups reducing their total wake time. Results from pretreatment-posttreatment contrasts further revealed that patients in the cognitive behavior therapy group and in the group that received combined cognitive behavior therapy and medication taper reduced their total wake time significantly more than those in the medication taper group (all p<0.002).
Comparisons of posttreatment with 3- and 12-month follow-up data yielded significant time effects for sleep efficiency (F=3.49, df=2, 182, p=0.03), total sleep time (F=21.25, df=2, 185, p=0.0001), and sleep onset latency (F=3.72, df=2, 183, p=0.03). In all three conditions, sleep efficiency and total sleep time values were higher, and sleep onset latency values were lower at the 3- and 12-month follow-ups.
Polysomnography
Linear mixed models (for three time points only: pretreatment, posttreatment, and 12-month follow-up) were performed by using data on the same variables derived from polysomnographic recordings, as well as the percentages of time spent in the different sleep stages (stages 1–4 and REM sleep). Significant time effects were obtained for total sleep time (F=18.94, df=2, 124, p=0.0001) and percentages of stage 2 (F=14.75, df=2, 119, p=0.0001), stages 3 and 4 (F=6.64, df=1, 123, p=0.002), and REM (F=6.38, df=2, 121, p=0.002) sleep. A priori contrasts revealed pretreatment-posttreatment effects on total sleep time (F=36.12, df=1, 117, p=0.0001) and percentages of stage 2 (F=29.43, df=1, 113, p=0.0001), stages 3 and 4 (F=12.66, df=1, 121, p=0.0005), and REM (F=12.68, df=1, 103, p=0.0006) sleep. The percentages of stages 3 and 4 sleep and REM sleep were significantly increased from baseline to posttreatment, while total sleep time and the percentage of stage 2 sleep were reduced for the same period. There was no other significant effect for group or group-by-time interaction for this period. No follow-up effect was significant for any variable.
Clinical Outcome Ratings and Psychological Symptoms
Linear mixed models yielded a significant time effect for the total score on the Insomnia Severity Index completed by the patient (F=38.42, df=3, 186, p=0.0001) and completed by the significant other (F=26.94, df=3, 151, p=0.0001). There were no significant group or interaction effects. A priori contrasts revealed a significant pretreatment-posttreatment effect for scores on the Insomnia Severity Index completed by the patient (F=94.23, df=1, 183, p=0.0001) and a significant group-by-time interaction effect (F=3.07, df=2, 183, p=0.05). Patients in all three conditions scored significantly lower at posttreatment than at baseline. A significant pretreatment-posttreatment effect was also obtained for the score on the Insomnia Severity Index completed by the significant other (F=66.19, df=1, 150, p=0.0001), but there was no group or interaction effect. For both versions of the Insomnia Severity Index, there was no further significant change from posttreatment to follow-ups. All three group means remained in the subclinical range (score <14) or below the threshold (score <8) for clinical insomnia.
All group means for the Beck Anxiety Inventory and Beck Depression Inventory were below 12 at baseline, suggesting no or minimal anxious and depressive symptoms. Linear mixed models yielded a significant time effect for the total score on the Beck Anxiety Inventory (F=6.14, df=3, 178, p=0.0001) and Beck Depression Inventory (F=18.53, df=3, 185, p=0.0001). A priori contrasts revealed significant reductions of depression (Beck Depression Inventory) and anxiety symptoms (Beck Anxiety Inventory) from baseline to posttreatment (F=16.83, df=1, 176, p=0.0001, and F=49.14, df=1, 184, p=0.0001, respectively). There was no other change on those variables at the 3- and 12-month follow-ups, with both scores remaining below the clinical thresholds (<10).
Linear mixed model analysis of the weekly total Clinical Institute Withdrawal Assessment—Benzodiazepines scores found a nonsignificant reduction in withdrawal symptoms over time for the medication taper group and the combined cognitive behavior therapy and medication taper group (F=1.81, df=9, 297, p=0.07). There was no significant effect for group or group-by-time interaction. There was no report of significant adverse events during medication tapering.
Treatment Attendance and Compliance
Subjects attended an average of nine therapy visits (SD=2.0; range=1–10). Analyses of blood samples obtained at baseline revealed that 100% of the participants were using benzodiazepine medication at the time of entering the study. The results of alcohol screening tests were all within the normal range. At posttreatment, all patients who reported not using benzodiazepine medication had negative results on the plasma benzodiazepine screening test. At the 12-month follow-up, one patient in the cognitive behavior therapy group who reported not using benzodiazepine medication at that time had a positive result on the plasma benzodiazepine screening test. The patients’ self-reports of benzodiazepine use were also compared with those obtained from their significant others’ reports. The percentages of agreement between the participants and their respective significant others were 95% at posttreatment, 82% at 3-month follow-up, and 87% at 12-month follow-up.
Discussion
The results indicate that a supervised medication tapering program and cognitive behavior therapy, singly or combined, are effective methods for reducing use of benzodiazepine medications among older adults with chronic insomnia. Despite regular and prolonged use, nearly two-thirds (63%) of the patients were drug-free within an average of 7 weeks, and the majority of the remaining patients also achieved a clinically meaningful reduction of benzodiazepine use. The absence of significant rebound insomnia during the withdrawal period was a positive outcome in itself. Sleep improvements, although modest initially, became more noticeable at follow-ups.
The combined approach, involving both structured medication tapering and a behavioral program for insomnia, was generally more effective than either of its components alone. For instance, there were more drug-free patients posttreatment in the combined intervention group (85%) than in the medication taper group (48%) and the cognitive behavior therapy group (54%); also, the frequency of medicated nights was near zero in the combined intervention group, compared with about twice a week in the groups that received a single intervention.
The two groups receiving an insomnia-specific intervention (i.e., cognitive behavior therapy or combined cognitive behavior therapy and medication taper) had modest but significant sleep improvements (i.e., reductions of total time awake), according to the patients’ sleep diaries, from baseline to posttreatment. Additional improvements in sleep efficiency and sleep onset latency became noticeable at the follow-up assessments; in addition, the initial reduction of sleep time noted at posttreatment was regained by the 3-month follow-up and maintained later. These results highlight the importance of incorporating clinical procedures that specifically target insomnia during benzodiazepine discontinuation; also, they emphasize the need to inform patients in clinical practice of the potential delay, even after benzodiazepine discontinuation, before they experience sleep improvements.
The benzodiazepine reductions achieved during the initial intervention were generally well maintained at the follow-ups. For instance, the proportion of drug-free subjects remained above or near 50% at the follow-up assessments, even given the conservative assumption that subjects lost to follow-up (i.e., dropouts) had resumed taking medication. In addition, the average nightly use of medication remained significantly below initial use. The early advantage of the combined intervention was not entirely sustained over time; although the outcome on all three medication variables was still better for the combined condition, the group differences were no longer statistically significant at follow-ups. This gradual loss of group differences over time, particularly between the medication taper and combined cognitive behavior therapy and medication taper conditions, may be due to subjects’ attrition (and reduced power) or to decreased adherence to the behavioral procedures in the combined intervention. This later explanation would suggest the need to incorporate periodic booster consultation sessions over time in order to maintain compliance with clinical procedures for insomnia and for minimizing relapse regarding benzodiazepine use.
The patients reported few withdrawal symptoms. Because rebound insomnia and anxiety are classic symptoms associated with abrupt benzodiazepine withdrawal
(54), this finding is most likely explained by the very gradual and flexible schedule of the medication tapering program. Although previous studies have shown that the addition of psychological treatment reduced withdrawal symptoms relative to standard medication tapering alone among patients using benzodiazepine medication for anxiety
(34,
35), there was no such difference between the medication taper and the combined approach in the current study, possibly because of a “floor effect.”
Several factors may limit the generalizability of the findings. First, all of the participants were white, healthy, relatively “young” older adults who were willing and motivated to discontinue their benzodiazepine medication. With one or two exceptions, the participants were not abusing benzodiazepine medication in the sense of exceeding the recommended dose
(21,
40). Rather, they were prolonged users of what might be considered a low therapeutic dose of benzodiazepine (average dose of 10 mg of diazepam equivalent per night). Yet, they had already attempted unsuccessfully to discontinue taking benzodiazepine medication an average of six times before this study; as such, they met the criteria for hypnotic-dependent insomnia
(20) and were representative of patients typically seen in clinical practice
(32). Nonetheless, the nature of the study group precludes generalization of the findings to older patients with chronic medical or mental health problems or to those who are chronic users and do not wish to discontinue or even reduce their benzodiazepine medication.
Despite these limitations, the present findings have direct implications for clinical practice. First, they show that regular and chronic users of benzodiazepine medication for sleep can be successful in discontinuing their medications without altering the quality of their sleep or enhancing anxiety and depressive symptoms. The results indicate that a supervised medication taper is a necessary component to guide patients in discontinuing benzodiazepine medications. This guidance alone may be sufficient for some patients, but the addition of formal cognitive behavior therapy specifically targeting insomnia and withdrawal symptoms appears to facilitate benzodiazepine discontinuation by minimizing sleep disruptions and anxiety that may arise or worsen during the medication tapering period. The nature and extent of this concurrent intervention may vary according to severity of withdrawal symptoms and sleep difficulties. For some patients, support and education about the transient nature of withdrawal symptoms and rebound insomnia may be sufficient. For others, behavioral correction of poor sleep habits may be particularly useful to minimize sleep difficulties, and cognitive therapy may be helpful in reducing apprehensions about drug discontinuation and in reappraising withdrawal symptoms as temporary and manageable
(22,
32,
55). These interventions can be implemented in most medical practices. Although the addition of cognitive behavior therapy may be more time consuming, previous studies suggest that cognitive behavior therapy, even an abbreviated program, can be implemented successfully in primary care medicine
(56–
58).
In summary, the present findings extend those from previous studies in documenting the efficacy of clinical procedures to facilitate benzodiazepine discontinuation
(27–
32). Additional placebo-controlled studies are needed to determine the essential therapeutic components of those interventions and to evaluate the benefits of relapse prevention training for maintaining abstinence over time. Longitudinal studies are also warranted to identify risk factors for prolonged use of hypnotic medications and predictors of relapse or successful discontinuation. It would also be important to examine the broader effects of benzodiazepine discontinuation on sleep, cognitive functioning (memory), and quality of life
(59–
61).