Major depression is an important public health problem that affects about 16% of the population at some time in their lives
(1). Depression is therefore the most common disorder in this country that is likely to be associated with major morbidity as well as loss of economic productivity
(2). The risk of recurrence of depression increases with repeated episodes. For instance, the risk of having another depressive episode after a single episode is about 50%, whereas with a past history of two or more episodes, the risk of having a recurrence of depression is greater than 90%. One possible explanation for this pattern of recurrence is that changes in the brain that are caused by depressive episodes may lead to an increased risk of recurrence. However, in spite of the importance of depression for our society, little is known about changes in the brain that may lead to depressive episodes and promote the recurrence of depression.
Animal models of depression, such as exposure to chronic uncontrollable stress, demonstrate long-term alterations in neurophysiology
(3–
6). Glucocorticoids such as cortisol, which are released during stress, were associated in some studies with damage to neurons
(7–
10) and inhibition of neurogenesis
(11,
12) in the hippocampus, a brain area involved in learning and memory, with associated deficits in hippocampal-based memory functioning
(13,
14). Hypercortisolemia is a consistent finding in a subgroup of subjects with severe depression
(15–
21) that may be related in part to hippocampal dysfunction
(22,
23). Subjects with depression also show deficits in performance on hippocampal-based verbal declarative memory tasks, including paragraph delayed recall and word list learning
(24–
29), that may be mediated by the effects of cortisol on the brain
(30,
31). The hippocampus has important connections with the prefrontal cortex, which has also been implicated in depression
(32,
33). The areas of the prefrontal cortex that have been implicated in depression include the dorsolateral prefrontal cortex (involved in working memory) and medial prefrontal cortex (including the anterior cingulate and orbitofrontal cortex).
Imaging studies have implicated the hippocampus and prefrontal cortex in the mediation of depressive symptoms
(34–
36). Magnetic resonance imaging studies of subjects with depression showed smaller hippocampal volumes and other abnormalities in hippocampal structure in some studies
(37–
45), but not others
(46,
47), and smaller subgenual (anterior cingulate) cortical
(48) and orbitofrontal cortical
(49,
50) volumes. Multiple studies of blood flow and/or metabolism, using positron emission tomography (PET) or single photon emission computed tomography, in subjects with untreated depression have shown low functioning in the left
(51–
54) and bilateral
(55–
57) dorsolateral prefrontal cortex and in the medial prefrontal cortex/anterior cingulate/orbitofrontal cortex
(48,
57–62). Induction of depressive symptoms with reduction of brain serotonin
(32) or norepinephrine
(63) levels resulted in decreased metabolism in the dorsolateral prefrontal cortex, orbitofrontal cortex, and thalamus. In summary, the imaging findings in depression to date are consistent with dysfunction of the hippocampus and prefrontal cortex (specifically anterior cingulate) in depression. A gap in the literature has been the use of specific probes, such as memory encoding, of hippocampal and frontal cortical functioning in depression.
The PET studies performed to date in healthy human subjects have not consistently shown activation of the hippocampus during performance of verbal declarative memory tasks. Some studies have shown activation with tasks such as stem completion
(64–
66), although a larger number of studies have not
(67–
75). Factors such as the success of retrieval
(69,
76–78) and emotional valence
(74) have been suggested as factors that could affect hippocampal activation. More consistent hippocampal activations has been shown for encoding (as opposed to retrieval) tasks
(79–
85). We have found hippocampal activation in normal subjects with a paragraph encoding task
(86). The paragraph encoding task involved encoding of complex and integrated information that uses the integration functions hypothesized as a role for the hippocampus
(87). This type of cognitive function is also classically impaired in patients with known hippocampal lesions
(88). Deficits in paragraph recall have been associated with depression. We therefore used paragraph encoding as the verbal declarative memory task in the current study, whose purpose was to use a verbal declarative memory encoding task as a probe of hippocampal and anterior cingulate functioning in subjects with midlife depression. We hypothesized that subjects with depression would show less activation of the hippocampus and anterior cingulate during the declarative memory task than would healthy comparison subjects.
Method
Subjects
Twenty-seven men and women participated in the study, including nine subjects without a history of major depression and 18 with midlife depression. The subjects included six healthy women and three healthy men and 13 women and five men with major depression. The project was approved by a local human investigation committee. All subjects were recruited through newspaper advertisement. The diagnosis of major depression was established with the Structured Clinical Interview for DSM-IV (SCID)
(89). The subjects were also evaluated with the Hamilton Depression Rating Scale, a validated instrument for measurement of depression severity
(90). All subjects gave written informed consent for participation, were free of major medical illness on the basis of history and physical examination, laboratory testing, and electrocardiogram, were not actively abusing substances or alcohol (in the past 6 months), and were free of all medications for at least 4 weeks before the study. The subjects did not stop taking medication for the purpose of participating in the study. Subjects with a serious medical or neurological illness, an organic mental disorder or comorbid psychotic disorder or posttraumatic stress disorder (PTSD), a history of childhood trauma as measured with the Early Trauma Inventory
(91), a current or past history of alcohol or substance abuse or dependence, retained metal, or a history of head trauma, loss of consciousness, cerebral infectious disease, or dyslexia were excluded. There was no difference in age between the healthy subjects (mean=38 years, SD=2) and the subjects with major depression (mean=43 years, SD=2) (t=1.03, df=25, p=0.31). There was also no difference in the number of years of education between the healthy subjects (mean=16, SD=2) and the subjects with depression (mean=16, SD=2) (t=0.76, df=25, p=0.45). All subjects were right-handed.
All of the depressed subjects had current and lifetime unipolar major depression. The current level of depressive symptoms as measured with the Hamilton depression scale was 33 (SD=9) in the depressed group. Three of the subjects (17%) had depression with melancholia; none had atypical depression. Two (11%) of the 18 depressed subjects fulfilled the criteria for a lifetime history of dysthymia, and one (6%) had a current history of dysthymia based on the SCID. Five (28%) of the 18 had new-onset major depression; the rest (72%) had recurrent major depression. The comparison subjects did not have a history of psychiatric disorder as measured with the SCID.
PET Scanning Methods
Each subject was scanned on a single day in conjunction with verbal memory tasks. The subjects underwent PET scanning with four activations (two control tasks and two memory encoding tasks). Consecutive PET scans were separated by 10 minutes. H2[15O] was prepared on-site in a cyclotron. An intravenous infusion of normal saline was started to permit the bolus injection of H2[15O]. Each subject was scanned with his or her eyes open in a dimly lit room. The subject was placed in the scanner with the head held in a head holder to minimize motion. The head was positioned with the canthomeatal line parallel to the external laser light. After positioning within the camera gantry, a transmission scan of the head was obtained by using an external 67Ga/68Ge rod source. This data were used to correct emission data from attenuation due to overlying bone and soft tissue. The subject received a 30-mCi intravenous bolus of H2[15O] for each of the four scans. Each condition lasted 60 seconds, with the PET scan acquisition beginning at the initiation of the condition and ending after 60 seconds.
The subjects were initially instructed that this was a test of how “the brain processes information” and were not told that it was a test of memory. Before the first two scans they were read a list of 10 pairs of words for one minute and asked to count the number of times they heard a word that contained the letter “D” (a control condition providing poorly encoded words). They were then read a second list of 10 different word pairs for 1 minute and again asked to count the number of times they heard the letter “D.” These words were neutral in content and were all concrete nouns in common usage in the English language. Methods related to the development of these words, including ratings of emotional content, were previously published
(74). The subjects then underwent PET imaging for scan 1 while they were asked to remember the poorly encoded words. This was repeated for scan 2 for the second list. Before scan 3, the subjects were told that a paragraph would be read to them during the next scan, and they were asked to remember it and to form an image of the scene in their mind during the scan (deep encoding). The paragraph read to them during scan 3 contained neutral content. Five minutes later the subjects were asked to remember the paragraph, and the accuracy of their recall was scored. This procedure was repeated for scan 4.
Image Analysis
The images were reconstructed and analyzed on a SunSparc Workstation by using statistical parametric mapping (SPM 96). The images for each patient set were realigned to the first scan of the study session. The mean concentration of radioactivity in each scan was calculated as the area-weighted sum of the concentration of each slice and adjusted to a nominal value of 50 ml/minute per 100 g. The data were transformed into a common anatomical space by using the Montreal Neurological Institute template
(92) and were smoothed with a three-dimensional Gaussian filter to 16 mm full-width at half maximum. Regional blood flow, with global blood flow as a covariate, was compared in the control and deep encoding conditions in the subjects with depression and the comparison subjects. The interaction between group (depressed versus comparison subjects) and condition (control condition versus deep paragraph encoding) was also examined. Repeated measures of cerebral blood flow at rest have shown variability of 2.3% (SD=8.7%) within subjects
(93) and 4.5% from our site
(94). Statistical analyses yielded image data sets in which the values assigned to individual voxels corresponded to t statistic values
(95,
96). The statistical images were displayed with values of z score units. A threshold z score of 2.58 (p<0.005) was used to define areas of activation within regions included in our hypothesis. The threshold z score corresponds to a p value of <0.001 for a one-tailed t test. Since the current study used an a priori hypothesis, this justifies the use of a one-tailed test. Locations of areas of activation were identified as the distance from the anterior commissure in millimeters, with x, y, and z coordinates, based on a standard stereotaxic atlas
(97).
The relationships between behavioral and brain measures were assessed by using Spearman correlations. Analysis of variance was used to assess the difference in behavioral measures and memory performance between subjects with and without depression.
Results
There were no significant differences between the subjects with depression and the comparison subjects in paragraph recall scores for either the first strongly encoded paragraph (mean=24, SD=7, versus mean=28, SD=8; t=1.43, df=24, p=0.17) or the second one (mean=26, SD=9, versus mean=30, SD=6; t=1.05, df=25, p=0.31). There were also no differences in recall of the weakly encoded memory material.
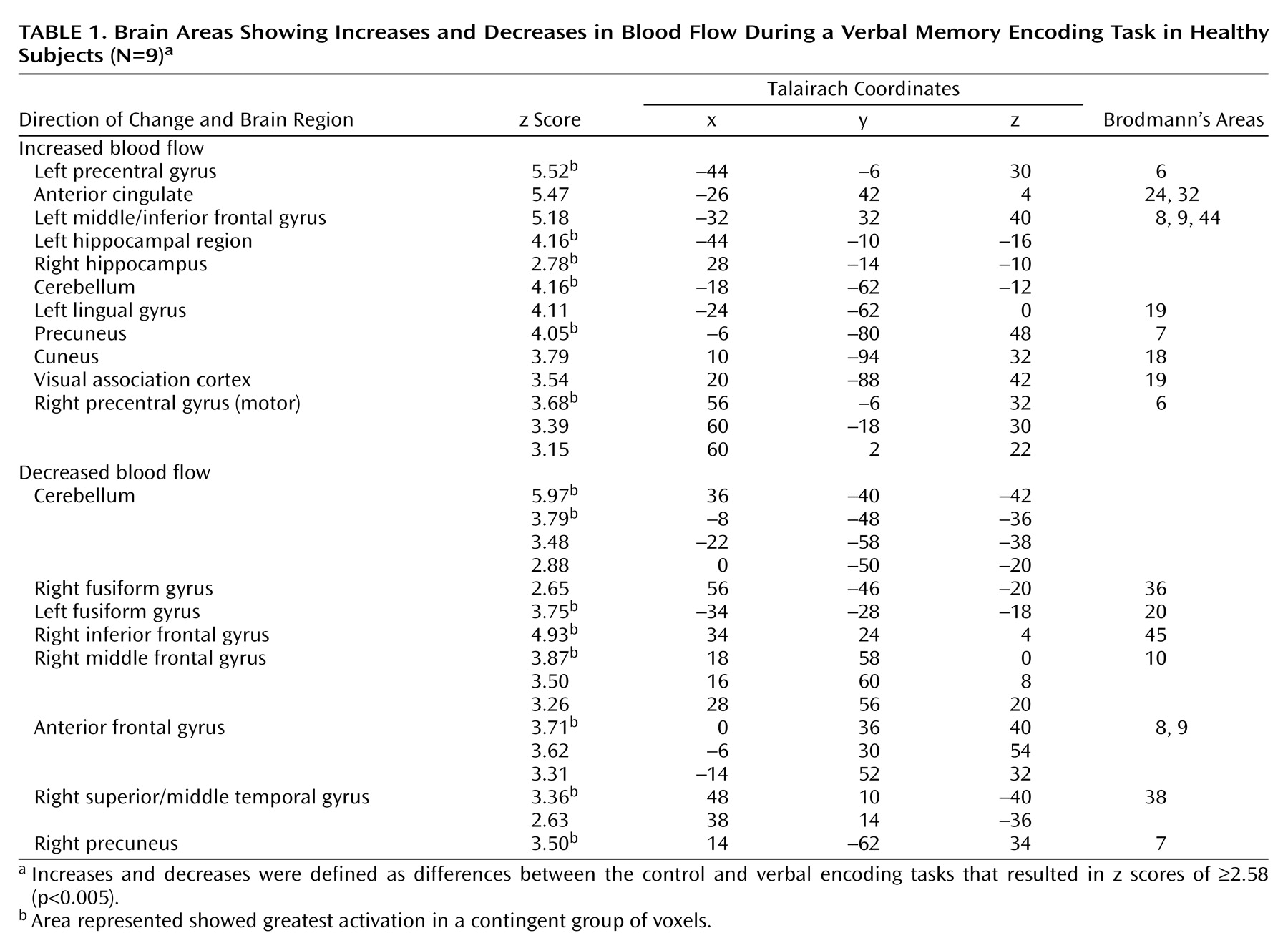
Memory encoding of a paragraph by the healthy subjects resulted in increased blood flow in the hippocampus, anterior cingulate, cerebellum, lingual gyrus, precuneus, cuneus, visual association cortex, motor cortex, and inferior/middle frontal gyrus. Paragraph encoding resulted in a decrease in blood flow in the right middle/inferior frontal gyrus, anteromedial prefrontal cortex, bilateral fusiform gyrus, cerebellum, and right middle temporal gyrus (
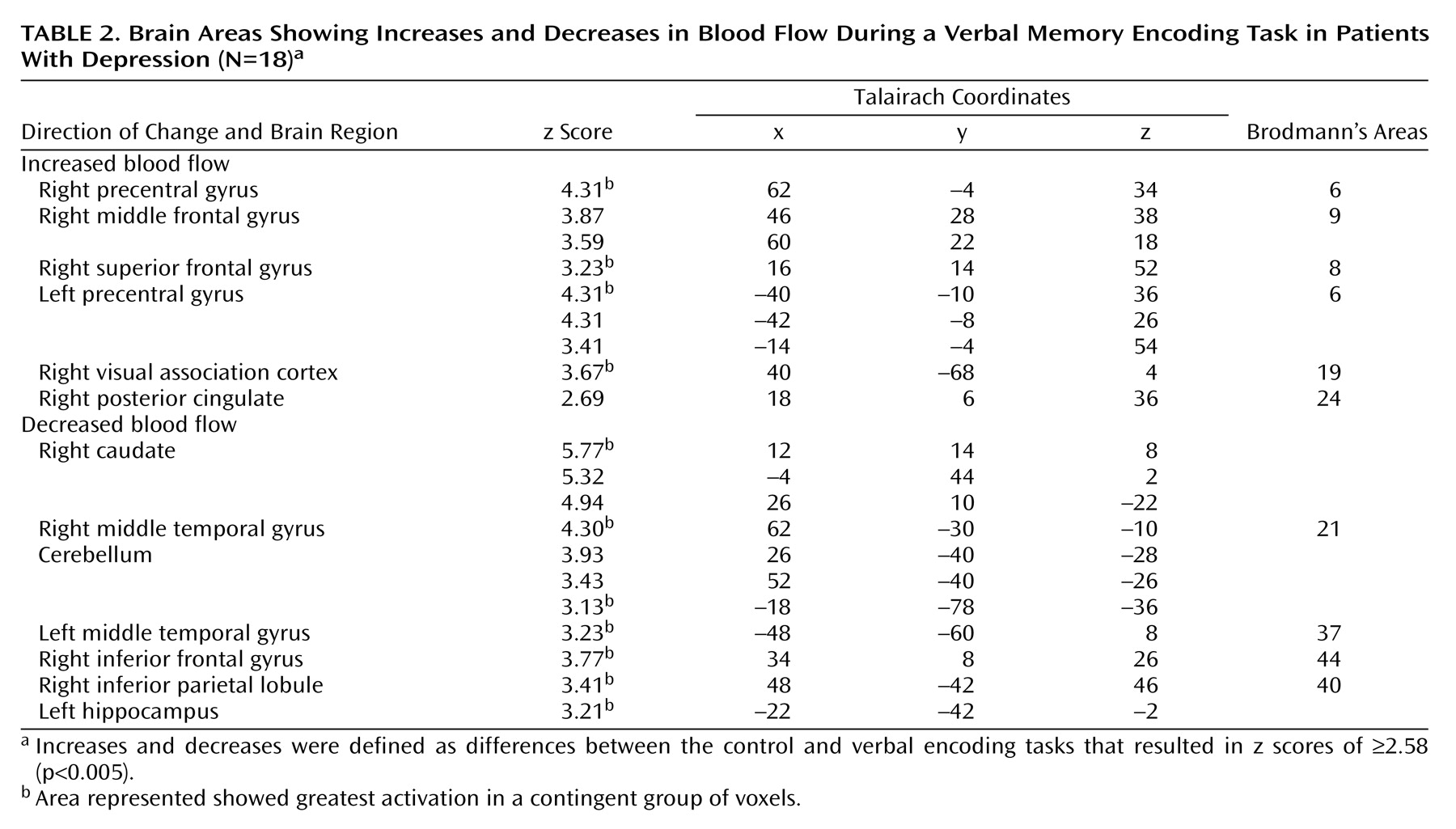
Table 1). Memory encoding by the subjects with depression resulted in an increase in blood flow in the bilateral precentral gyrus (motor cortex), right middle and superior frontal gyrus, right visual association cortex, and right posterior cingulate (
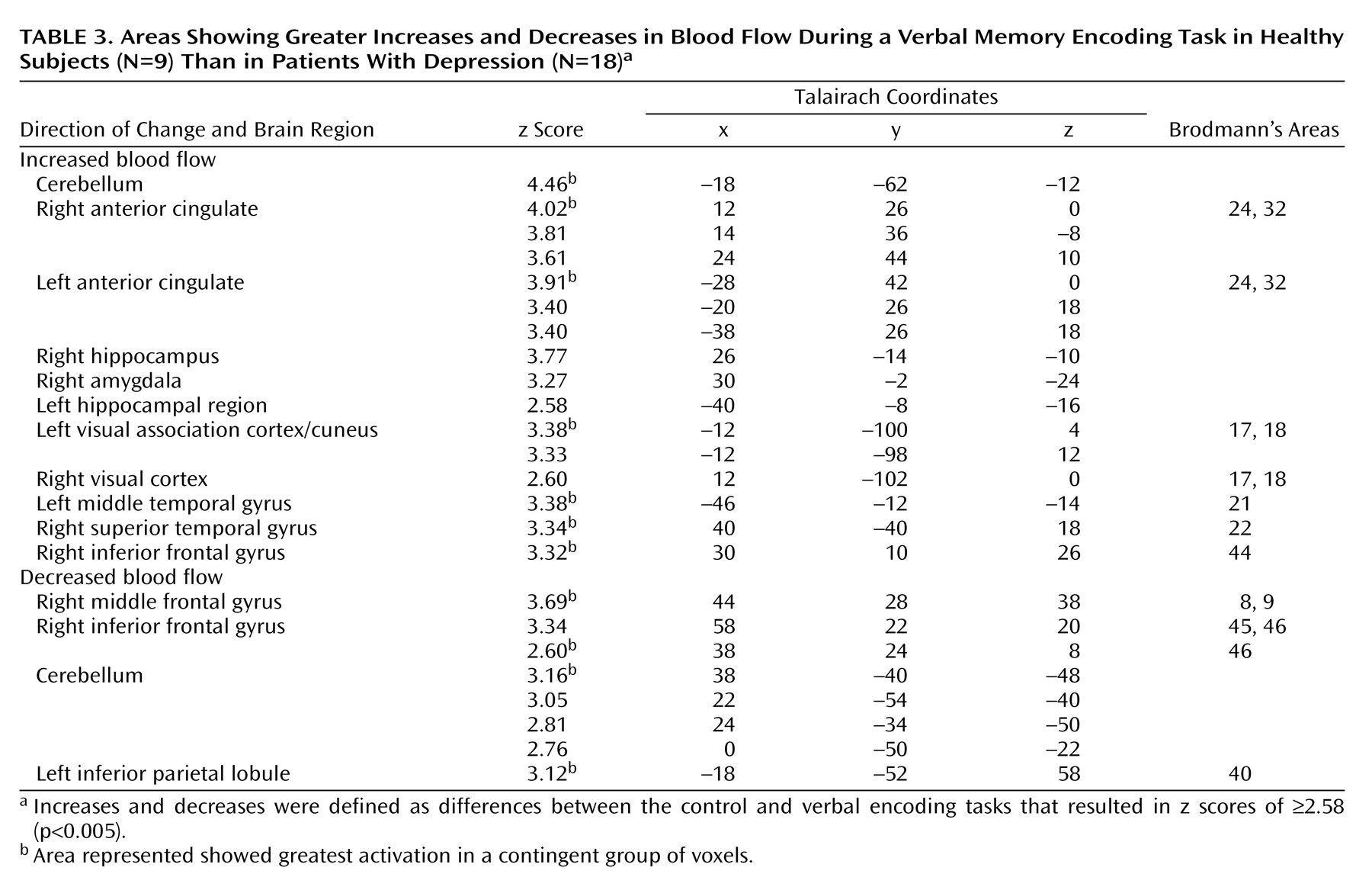
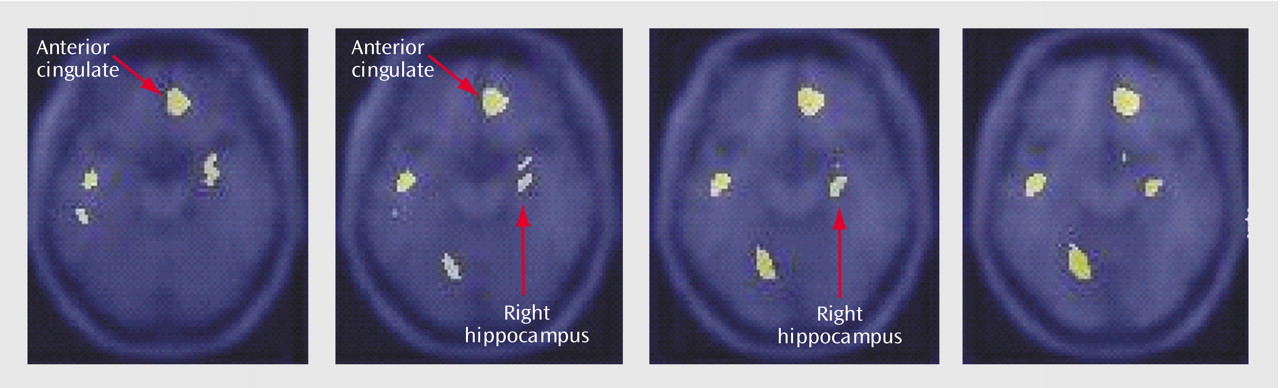
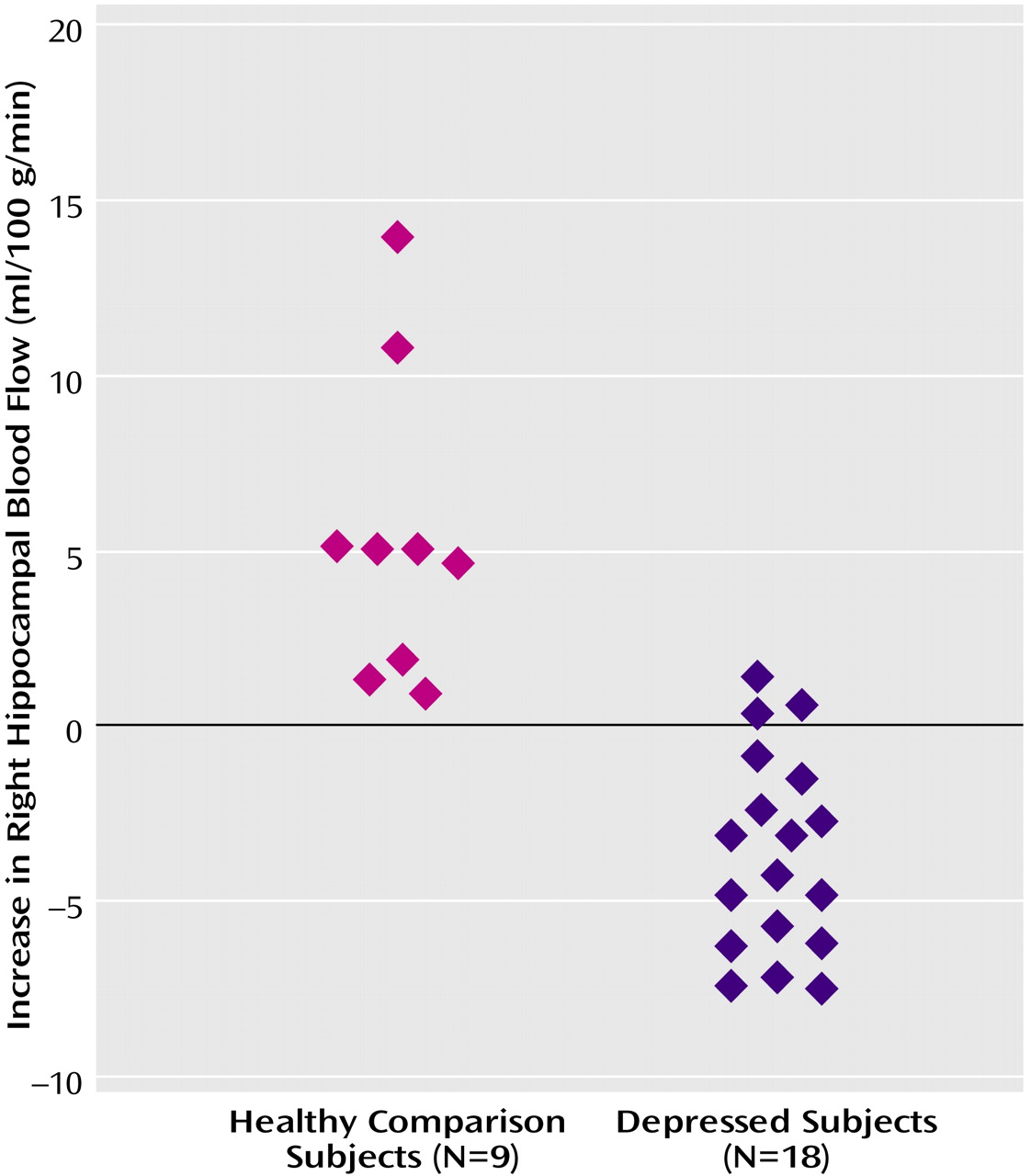
Table 2). Memory encoding resulted in a decrease in blood flow in the depressed subjects in the right caudate, bilateral middle temporal gyrus, cerebellum, right inferior frontal gyrus, right inferior parietal lobule, and left hippocampus. Comparison of the healthy subjects with the depressed subjects showed that the healthy subjects had greater increases in blood flow during memory encoding in the cerebellum, bilateral anterior cingulate, right hippocampus and amygdala, left hippocampal region, right cuneus, left visual association cortex, left middle temporal gyrus, right superior temporal gyrus, and right inferior frontal gyrus (
Table 3,
Figure 1,
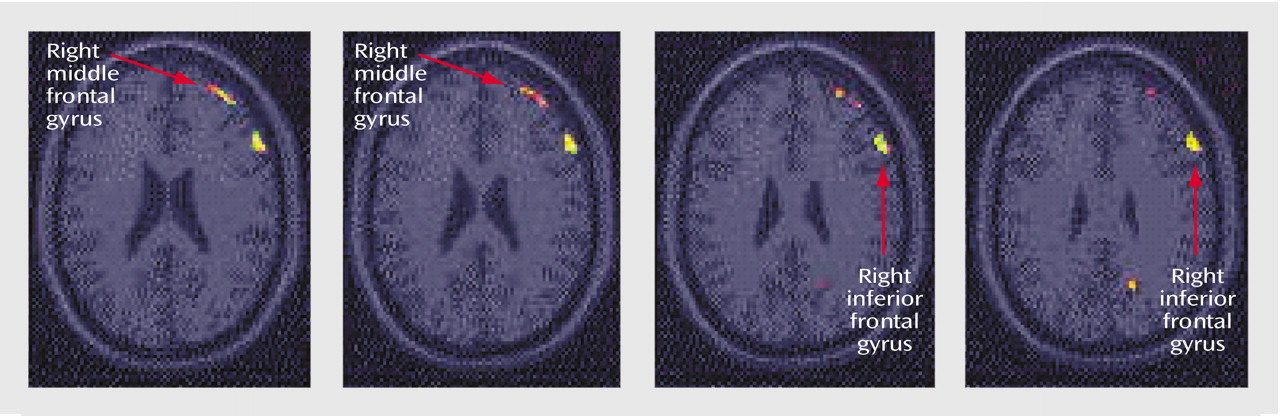
Figure 2). The healthy subjects showed greater decreases in blood flow with memory encoding in the right middle and inferior frontal gyrus, cerebellum, and left inferior parietal lobule (
Table 3,
Figure 3).
There was no correlation between hippocampal activation and several demographic factors, including age and sex, in the depressed or nondepressed subjects. There was no relationship between level of depression as measured by the Hamilton depression scale and hippocampal activation in the depressed subjects. There were also no differences in hippocampal activation between the subjects with new-onset depression and those with recurrent depression.
Discussion
Memory encoding resulted in greater hippocampal and anterior cingulate activation in healthy subjects than in subjects with currently untreated midlife depression. These findings are consistent with the hypothesis of hippocampal and anterior cingulate dysfunction in depression. The use of a verbal memory encoding task as a specific probe of hippocampal and prefrontal functioning provides evidence that converges with findings from a number of structural and functional baseline imaging studies that suggest dysfunction in the hippocampus and prefrontal cortex in depression.
The network of brain regions activated by memory encoding differed between the subjects with depression and the healthy subjects. For example, there was greater activation of the right middle and inferior frontal gyri in the subjects with depression. These subjects may have relied on these areas to a greater degree because of dysfunction in the hippocampus and anterior cingulate. The right inferior frontal gyrus has been hypothesized to be involved in the effort of retrieval, but not necessarily in successful retrieval. It is of interest that the depressed subjects relied more on the right middle frontal gyrus while the nondepressed subjects used the left middle frontal gyrus in encoding. There were no differences in performance of the paragraph recall task, however, between the subjects with and without depression. This suggests that the differences in brain activation were not primarily related to differences in performance.
Memory encoding in the healthy subjects involved recruitment of an extended network of brain regions similar to those shown by prior imaging studies of the neural correlates of memory. Brain regions involved in memory encoding by the healthy subjects in the current study that have been found to be activated in prior studies of the neural correlates of declarative memory include the precuneus
(72,
73,
98), cuneus
(65,
74), left inferior/middle frontal gyrus
(65,
69,
70,
72,
73), and precentral gyrus
(70,
73,
74). The inferior/middle frontal gyrus portion of the prefrontal cortex is felt to be involved in verbal memory encoding and/or retrieval
(99), as well as verbal processing
(100,
101) and working memory
(102,
103). In prior PET studies of normal subjects, the anterior cingulate
(65,
68–
70,
72,
74,
98,
99) has been consistently associated with verbal memory retrieval, while some studies (but not all) showed hippocampal activation
(65,
67,
76). The depressed subjects in this study shared with the healthy subjects recruitment of the visual association cortex.
We did not find a correlation between hippocampal activation and memory performance in the patients with depression. This is in contrast to the findings in a study using a word generation task in patients with geriatric depression
(61), which showed hippocampal (and anterior cingulate) deficits that correlated with deficits in baseline memory scores (outside of the scanner) derived from the Dementia Rating Scale. That study had several important differences from the current one, including the age of the study group, differences in the cognitive task, and the fact that memory ratings did not correspond to the task performed during the imaging study.
There are several possible explanations for the findings of the current study. Hypercortisolemia associated with stress and/or depression may lead to dysfunction of the hippocampus during the performance of verbal declarative memory tasks by subjects with depression. We did not obtain blood samples to test this hypothesis, however, and our prior studies did not indicate a correlation between hippocampal volume and a single plasma cortisol measurement
(37,
104). Other factors linked to stress, such as decreased brain-derived neurotrophic factor, may lead to hippocampal dysfunction. Stress may independently lead to neurochemical alterations resulting in hippocampal dysfunction and depression, or stress may cause hippocampal dysfunction, which then leads to both the emotional dysregulation and cognitive disturbances seen in depression. It is also possible that altered hippocampal function from birth is a risk factor for the development of depression, rather than a result of stress and/or depressive episodes. With regard to prefrontal functioning, there is emerging evidence that early life experience may influence neurogenesis within the prefrontal cortex. Alternatively, a circuit within the hippocampus may be affected in depression, or alterations in functioning from birth may account for changes in functioning in this area among subjects with depression.
There are several limitations of the current study that should be taken into consideration. The reason for having a fixed order of tasks, with the control tasks followed by the deep encoding tasks, was that once the subjects were aware that this was a test of memory they would remember words in spite of the instructions. However, this design may have led to order effects (e.g., subjects doing better as they went along). Also, the control task was not well matched to the encoding task. However, since the same protocol was applied to the depressed and nondepressed subjects, this should not explain differences across patient groups. The statistical parametric mapping data analysis technique has inherent limitations. For instance, images are transformed to a common anatomical space. Since individual subjects have different brain sizes, this may affect the final results. Also, the assessment of multiple regions of the brain may lead to false results related to multiple comparisons.
The proposed model of an abnormal circuit involving the hippocampus and prefrontal cortex is similar to that proposed for other disorders, including schizophrenia and PTSD. A number of studies have shown abnormalities in structure and functioning in the hippocampus and the functionally connected medial prefrontal cortex in schizophrenia
(105–
107). These findings raise the question of the specificity of circuit abnormalities in depression versus other psychiatric disorders. Dysfunction in these areas may represent the neural correlate of specific aspects of the disorder, such as hippocampal dysfunction that mediates cognitive abnormalities common to, for instance, both depression and schizophrenia, while abnormalities of the medial prefrontal cortex underlie both attentional deficits and affective blunting, which are common to both disorders. At this time it is not clear why similar circuits should subsume psychiatric disorders that are clearly different. One possibility is that although the neuroanatomical substrates may be similar, there may be differences in neurochemical functioning within the circuits that mediate differential expression of the disorders.