Disequilibrium and gait peculiarities were featured in classic descriptions
(1,
2) of patients with schizophrenia, even before the advent of neuroleptics, and are detectable with clinical neurological assessment
(3–
5). Ataxia of stance and gait is also salient behavioral sequelae to chronic alcohol abuse
(4,
6,
7) and may arise from compromise of the anterior superior cerebellar vermis (postmortem [
6,
8,
9] and in vivo
[10]).
Comorbidity of ataxia with alcohol use disorders is highly prevalent in schizophrenia (e.g., references
11,
12,
13) and has an adverse impact on schizophrenia’s clinical course (for reviews, see references
14,
15). Such comorbidity exacerbates existing deficits of brain volume in prefrontal and anterior temporal gray matter
(16) and produces deficits in the anterior superior cerebellar vermis
(17) and pons
(18), even in schizophrenia patients with remote histories of alcoholism and low levels of lifetime alcohol consumption relative to alcoholic patients without schizophrenia. Given this heightened vulnerability for patients comorbid for both schizophrenia and alcoholism, especially in the anterior superior cerebellar vermis, we assessed gait and balance by using quantitative ataxia tests to determine whether comorbid patients would show greater postural instability than patients with either condition alone.
Method
All subjects were men and gave written informed consent to participate in the research. The patients were recruited from a Veterans Administration medical center and included 10 with a DSM-III-R axis I diagnosis of schizophrenia only, 10 comorbid for DSM-III-R-defined schizophrenia and alcohol dependence or abuse, and 24 with DSM-III-R-defined alcohol dependence only. No patient met criteria for any other axis I disorder, with the exception of two of the comorbid patients who had a history of past cannabis abuse.
The patients with schizophrenia were tested for gait and balance while inpatients; the patients with alcoholism were tested when returning for follow-up studies
(19,
20). Alcoholic patients reported a wide range of days of sobriety (1 to 1,994, median=204) at the time of testing. The comorbid patients also reported a wide range of days of sobriety (17 to 3,285, median=88). The length of sobriety did not differ between these groups (Mann-Whitney U=117, df=32, p=0.91).
All schizophrenia patients had been treated pharmacologically; when tested, five schizophrenia and seven comorbid patients were taking atypical antipsychotic medications, three schizophrenic and two comorbid patients were taking typical antipsychotic medications, one schizophrenic patient was temporarily unmedicated, and the medication status was unknown for one schizophrenic and one comorbid patient. Current symptom severity was evaluated in patients with schizophrenia by using the Brief Psychiatric Rating Scale (BPRS)
(21), administered by two raters with established reliability. Lifetime alcohol consumption was assessed by using a semistructured interview
(22–
25) in all patients with alcoholism and healthy comparison subjects, nine of the 10 comorbid patients, and nine of the 10 patients with schizophrenia only.
The healthy comparison group was recruited from the local community and comprised 27 men selected from a larger group of 61 men (e.g., reference
10) to age-match the patient groups. All had been screened to exclude any axis I disorder, substance abuse in the year before the study, and alcohol consumption of more than four drinks a day for over a month.
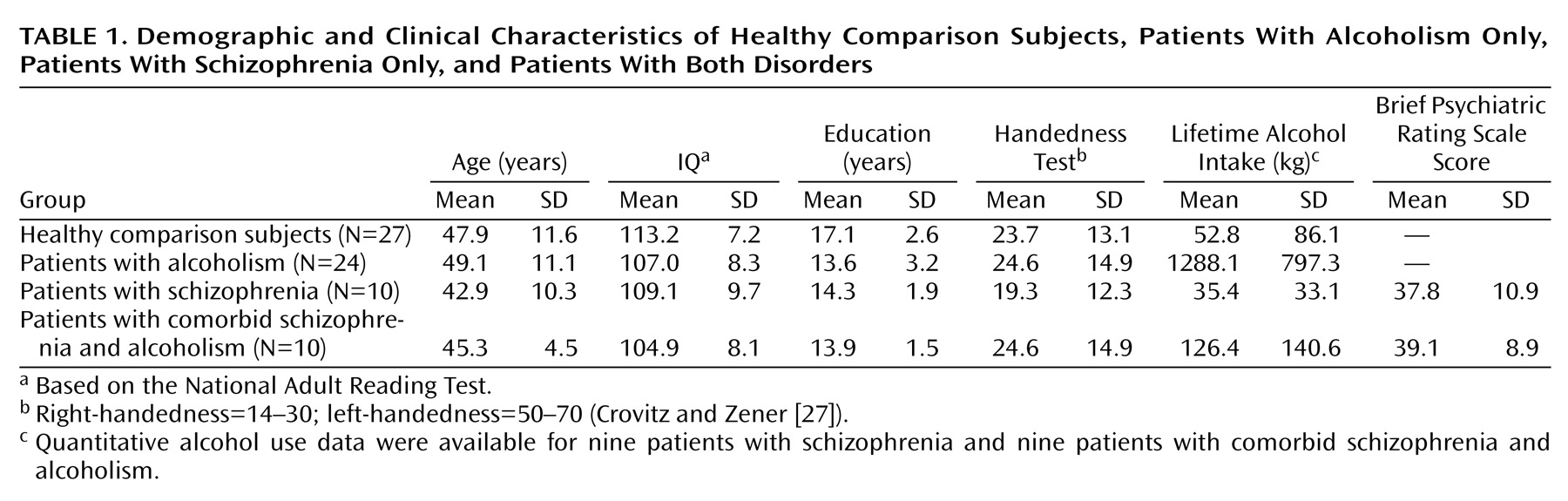
One-way analyses of variance (ANOVAs) of demographic variables (
Table 1) across all groups yielded significant differences in education (F=8.39, df=3, 67, p<0.0001), general intelligence (estimated with the National Adult Reading Test
[26]) (F=3.76, df=3, 67, p<0.05), and total lifetime consumption of alcohol (F=33.98, df=3, 65, p<0.0001) but not age (F=1.00, df=3, 67, p=0.42) or handedness (F=1.80, df=3, 65, p=0.15), measured quantitatively (27). Follow-up Scheffé tests (alpha=0.05) revealed that the group with alcoholism consumed more alcohol than all other groups. Differences between the schizophrenia and healthy comparison groups were not significant; the comorbid patients tended to drink more than the healthy comparison subjects (t=1.88, df=34, p<0.10) and the patients with schizophrenia (t=1.89, df=16, p<0.10). The three patient groups had equivalent years of education and National Adult Reading Test IQs. The comorbid group did not differ significantly from the schizophrenia group in BPRS scores.
Gait and static balance were assessed with the Walk-a-Line Ataxia Battery
(28), consisting of three parts, each performed first with eyes open and then with eyes closed. First, the subject stood with feet placed heel to toe and arms folded across the chest for 60-second trials (“stand heel to toe”). Next, the subject stood on one foot for 30-second trials (“stand on one foot”). Finally, the subject walked heel to toe for 10 steps (“walk heel to toe”). Each condition was performed twice unless the subject achieved a perfect score on the initial trial.
Because gait and balance performance can decline with age, we applied linear regression to data from the larger group of healthy comparison subjects spanning the adult age range (20 to 70 years) to derive age-corrected standardized z scores for each participant
(10). Values for each group reflect the extent to which it deviated from age norms. Group differences in performance for each composite were assessed with four-group, one- or two-way ANOVAs and follow-up Scheffé tests. Associations between variables were assessed with Pearson’s correlations and confirmed with Spearman’s tests because of small group sizes.
Results
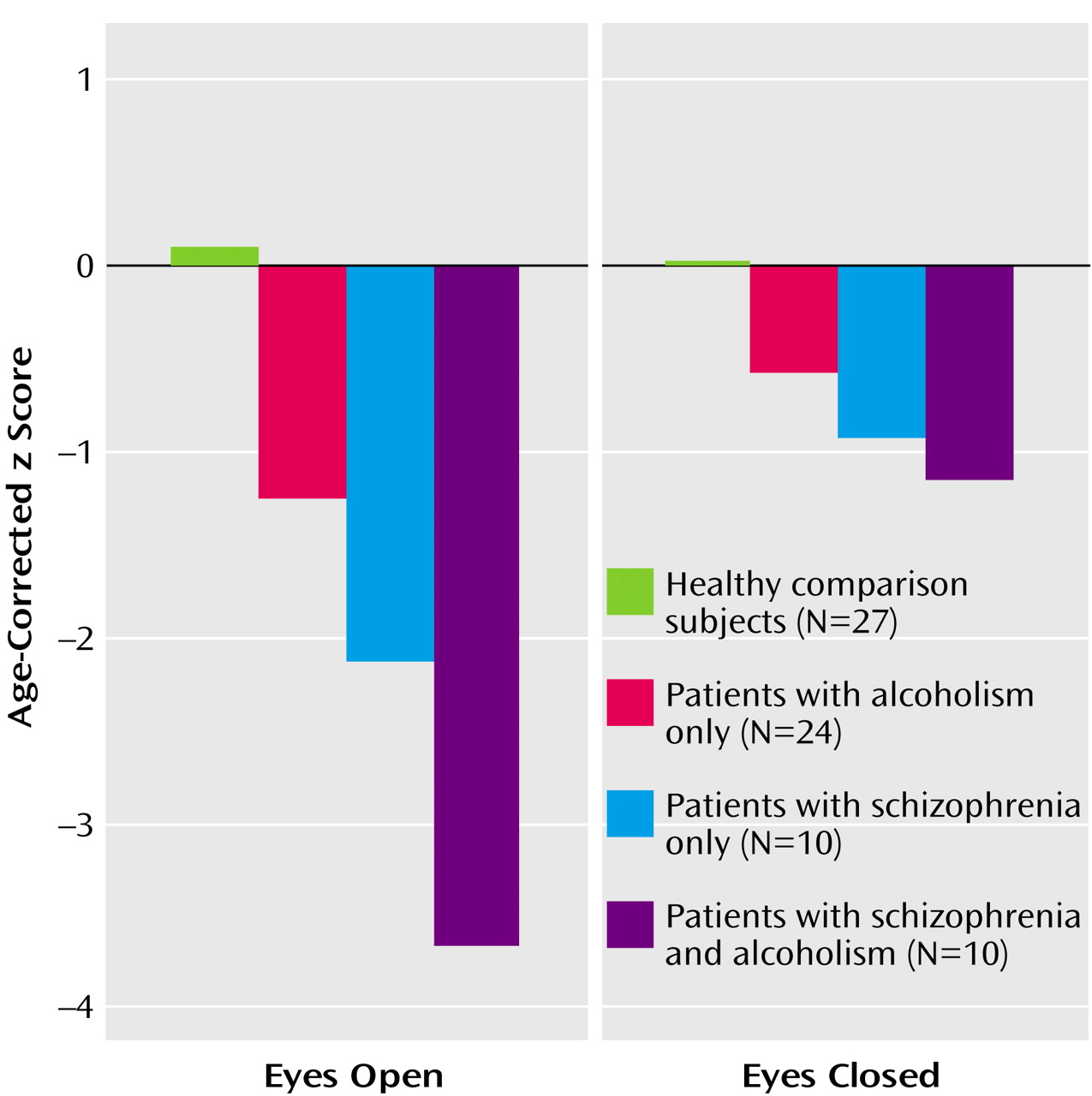
Composite scores for the eyes-open and eyes-closed conditions (
Figure 1) showed significant effects of group (F=15.97, df=3, 65, p=0.0001) and condition (F=13.24, df=1, 65, p=0.0005); the interaction (F=6.41, df=3, 65, p=0.0007) indicated greater deficits in all three patient groups with eyes open than with eyes closed. With one exception (alcoholics with eyes closed), follow-up tests showed that all patient groups had deficits in both conditions relative to the performance of the healthy comparison group, and the comorbid group had deficits relative to the alcoholic group.
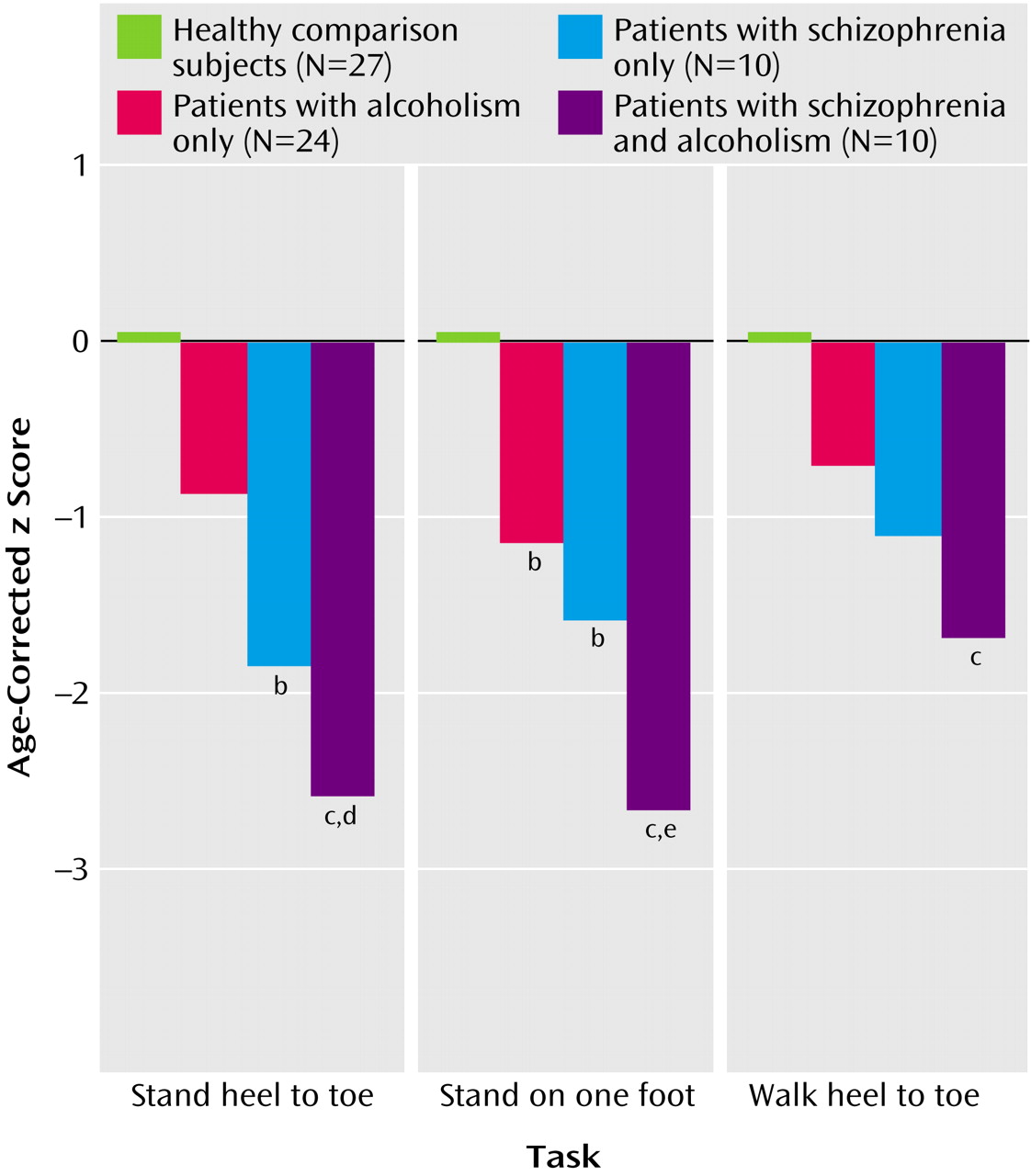
Composite scores for the three test parts—stand heel to toe, stand on one foot, and walk heel to toe (
Figure 2)—irrespective of visual condition, also showed effects of group (F=14.89, df=3, 132, p=0.0001) and test part (F=3.70, df=2, 132, p=0.028) but no interaction effect (F=1.70, df=2, 132, p=0.13). Scheffé tests indicated significantly lower scores than the comparison group on all three measures by the comorbid group, the two static balance measures by the schizophrenia group, and one measure (stand on one foot) by the alcoholic group. The comorbid group had significantly greater deficits than the alcoholic group for standing heel to toe and standing on one foot. Overall, the extent of the comorbid group’s deficit was the sum of the schizophrenia group’s and alcoholism group’s deficits.
Ataxia scores were not associated with lifetime alcohol consumption or length of sobriety in alcoholics or comorbid patients. Further, BPRS scores did not predict ataxia scores in either schizophrenia group, with or without alcoholism comorbidity. An ANOVA for factors of medication type (typical versus atypical) and diagnosis (schizophrenia versus comorbid) revealed no effect of medication or a medication-by-diagnosis interaction.
Discussion
This study identified deficits in balance and gait relative to age norms in patients with alcoholism, even following substantial periods of sobriety, as well as in patients with schizophrenia but without a history of alcoholism. The patients with schizophrenia who had also met lifetime criteria for alcoholism showed a compounded deficit (effect size across all tests ranged from SD=1.1 to SD=3.6), hence, greater than that observed in either condition alone (effect sizes ranging from SD=0.6 to SD=1.2 for alcoholism and SD=0.9 to SD=2.1 for schizophrenia). Comorbid patients were significantly more impaired than alcoholics, even though they had consumed only one-eighth of the alcohol over their lifetime relative to the nonpsychotic alcoholics. The group included only men; whether a similar pattern occurs in women remains to be studied.
The lack of statistically significant performance differences between comorbid patients and schizophrenia patients is consistent with a recent report also employing balance tests requiring standing in the Romberg position and walking heel to toe
(29). In that study, although the two schizophrenia groups did not show statistically significant differences in performance, the mean score of the comorbid group (where higher scores were in the impaired direction) was more than double that of the group with schizophrenia but without alcoholism.
Gait performance in comorbid and schizophrenia patients could have been affected by antipsychotic medication. Some studies have reported that patients taking neuroleptics perform significantly less well than untreated patients on fine motor coordination (e.g., reference
30). Others have reported that atypical neuroleptics are associated with better motor coordination than typical neuroleptics in patients with schizophrenia
(31). In this group, schizophrenia and comorbid patient groups did not differ in medication type, and there were no significant medication type effects or group-by-medication interactions. Thus, the relatively poorer performance of the comorbid patients than the schizophrenia patients is probably not solely a medication effect.
The additive untoward effect of alcoholism on an already compromised schizophrenic motor system
(4,
32) parallels our earlier reports of exacerbated regional brain volume in the prefrontal cortex
(16), cerebellar vermis, hemisphere gray matter
(17), and pons
(18) in patients comorbid for both conditions. A possible mechanism underlying this excessive postural instability is disruption of the cerebellar systems required for maintaining posture and balance, also present in schizophrenia-alcoholism comorbidity
(17). The results of the present study provide support for the working hypothesis that alcohol abuse, which occurs in about half the population of schizophrenia patients
(33), results in increased vulnerability of the brains of patients with schizophrenia to the exogenous toxin—alcohol. A manifestation of this increased vulnerability, readily demonstrated by standardized gait assessment, places comorbid patients at risk of falling. Contributing to this liability is a possible compromise of sensorimotor integration, implicated by the especially reduced ability of the comorbid group to stabilize balance with visual cues.