Dementia with Lewy bodies accounts for 15% to 25% of dementia cases
(1–
5). The condition is important because of the difficult clinical management issues presented by the high frequency of psychiatric symptoms
(6–
9) and the risk of severe neuroleptic sensitivity reactions
(10). Visual hallucinations are consistently reported to be more frequent in dementia with Lewy bodies than in Alzheimer’s disease, with most prospective studies indicating a frequency greater than 50%. Visual hallucinations are more persistent and appear earlier in the disease
(11,
12) in Lewy body dementia than in Alzheimer’s disease. Delusions are more common in dementia with Lewy bodies (frequency, 13%–75%) than in Alzheimer’s disease but are not more persistent
(12). The frequency of major depression is probably greater than 30% in dementia with Lewy bodies
(13,
14) and has been shown in some studies to be more frequent than in Alzheimer’s disease
(8,
9), but it is not more likely to persist
(12).
Patients who have dementia with Lewy bodies show a spectrum of pathology along the interface between Parkinson’s disease and Alzheimer’s disease. Seventy-five percent of patients with Lewy body dementia have many of the neuropathological features of Alzheimer’s disease, including senile plaques and neurofibrillary tangles
(1). Although typically the density of neocortical plaques is similar to that in Alzheimer’s disease
(1), the burden of tangles is less than in “pure” Alzheimer’s disease
(1,
13). When Lewy bodies occur in conjunction with Alzheimer pathology sufficient to meet the criteria of the Consortium to Establish a Registry for Alzheimer’s Disease (CERAD) for probable or definite Alzheimer’s disease
(15), neocortical neurofibrillary tangles are usually rare or absent, and tangles in the entorhinal cortex and hippocampus are intermediate between those of elderly comparison subjects and Alzheimer’s disease patients
(1,
13). Fewer than 40% of patients with Lewy body dementia meet the criteria of Braak and Braak
(16) for stage IV or higher neurofibrillary tangles and neuropil threads. The occurrence of Lewy bodies varies from restriction to the brainstem, or brainstem and limbic area, to widespread neocortical distribution
(14,
17).
A number of studies have compared clinical features in Alzheimer’s disease patients with and without Lewy bodies
(1,
11,
18–23), and they suggest that the presence of Lewy bodies is associated with visual hallucinations, delusions, and depression. To our knowledge, only one study has examined the persistence of psychiatric symptoms, suggesting an association with persistent visual hallucinations
(11). The majority of studies have been small case series, and many have used retrospective evaluation of clinical symptoms, with some potential for inaccurate symptom ascertainment and limited statistical power. In addition, several studies have compared patients with “pure” cases of Lewy body dementia (variously defined but usually including cases not meeting the CERAD criteria for probable or definite Alzheimer’s disease) to patients exhibiting significant Alzheimer’s disease pathology. One of these investigations focused on neuropsychiatric symptoms assessed with standardized scales, comparing 11 patients with pure cases of Lewy body dementia to 18 patients who exhibited mixtures of Lewy body dementia and Alzheimer’s disease pathology and 35 patients with pure Alzheimer’s disease
(13). The patients who had pure Lewy body dementia showed more hallucinations, delusions, and parkinsonism, whereas the patients with mixed cases resembled those with Alzheimer’s disease. In addition, one recent study examining the association of Lewy bodies with visual hallucinations across a pathological spectrum of parkinsonism, which included 29 patients with Lewy body dementia, showed a significant association between visual hallucinations and Lewy body density in the inferior temporal cortex
(24). These studies have mainly focused on Alzheimer’s disease patients with or without Lewy bodies, with little emphasis on the associations of psychiatric symptoms across the full range of patients with Lewy body dementia, including those with little Alzheimer’s disease pathology.
From an overview of this inconclusive literature, there is some indication that the presence of Lewy bodies in Alzheimer’s disease is associated with psychosis and that there are differences in clinical presentation between pure cases of dementia with Lewy bodies and those with concurrent Alzheimer’s disease pathology. The present study is based on an extensive study of prospectively studied patients who had autopsy-confirmed dementia with Lewy bodies.
Method
In total, we studied 112 patients with autopsy-confirmed dementia with Lewy bodies who had been prospectively assessed during life in one of four centers in Newcastle upon Tyne, U.K. (Newcastle Dementia Case Register, Institute for Ageing and Health); Oxford, U.K. (Oxford Project to Investigate Memory and Ageing, University of Oxford); London (Institute of Psychiatry Case Register); and London, Ont., Canada (Dementia Study Project, University of Western Ontario). At each center, consecutively assessed dementia patients who agreed to participate were enrolled. Diagnosis was made at autopsy according to the consensus criteria for a neuropathological diagnosis of dementia with Lewy bodies
(5). All patients meeting these neuropathological criteria were included. An additional 90 patients with neuropathologically diagnosed Alzheimer’s disease from the three U.K. centers were included as a comparison group. They comprised all prospectively studied patients from these centers with a neuropathological diagnosis of Alzheimer’s disease who had a Mini-Mental State Examination
(25) score higher than 0 at death (specification made to minimize differences between the two dementia groups at death, as far more of the patients with Lewy body dementia died before “end-stage” dementia). In total, autopsy tissue was collected from 445 prospectively assessed patients, 333 (75%) with Alzheimer’s disease and 112 (25%) with Lewy body dementia. At each of the centers, written consent was obtained for clinical evaluation, with written assent from each patient’s nearest relative after a full explanation of the study. Following death, written consent for autopsy was obtained from the next of kin in all cases. The study was approved by the human subjects research ethics committee in each of the centers.
A standardized psychiatric history
(26,
27) and a standardized assessment of cognitive function
(25,
28) were completed; different centers used different instruments. To estimate cognitive performance across centers, each patient was given a percentage score (the percentage of points scored by the patient out of those available on the schedule used), with a higher percentage indicating better performance.
A full neuropsychiatric evaluation was completed at each center with validated instruments
(29–
31). The diagnosis of depression was made by using DSM-III-R criteria but without the organic exclusion clause when dementia was the only relevant organic condition. Definitions of the presence and classification of psychotic features were taken from the criteria of Burns et al.
(32). At each center a standardized physical examination included a detailed evaluation of parkinsonism
(33). Repeat evaluations were undertaken at least annually until death. Symptoms were rated as persistent if they were present for at least 6 months continuously at some stage of the dementia (either present at two or more consecutive assessments or present for 6 months according to the patient’s history at the time of the initial evaluation). For the individuals who died before any follow-up assessment was completed, the determination of chronicity was made on the basis of a history of the continuous presence of the symptom for at least 6 months before the evaluation. This is the same definition of chronicity as applied in previous publications from the Newcastle group.
At each center, the neuropathological diagnosis of Alzheimer’s disease was made according to the CERAD protocol
(15), and the severity of plaques was determined by using the CERAD guidelines
(15). The staging method of Braak and Braak
(16) was used to quantify tangle pathology. The presence or absence of Lewy bodies was assessed in the brainstem and in limbic and neocortical areas as outlined in the consensus criteria for dementia with Lewy bodies
(5). The staging of Lewy body pathology followed the same consensus guidelines. The neuropathological diagnosis of dementia with Lewy bodies was hence independent of the severity of Alzheimer’s disease pathology, and patients with sufficient Lewy bodies in the key diagnostic areas were diagnosed as having dementia with Lewy bodies even in the presence of extensive plaque or tangle pathology. Lewy bodies were not evaluated in other areas, and so coincidental Lewy bodies in other regions, such as the amygdala, would not lead to a diagnosis of dementia with Lewy bodies.
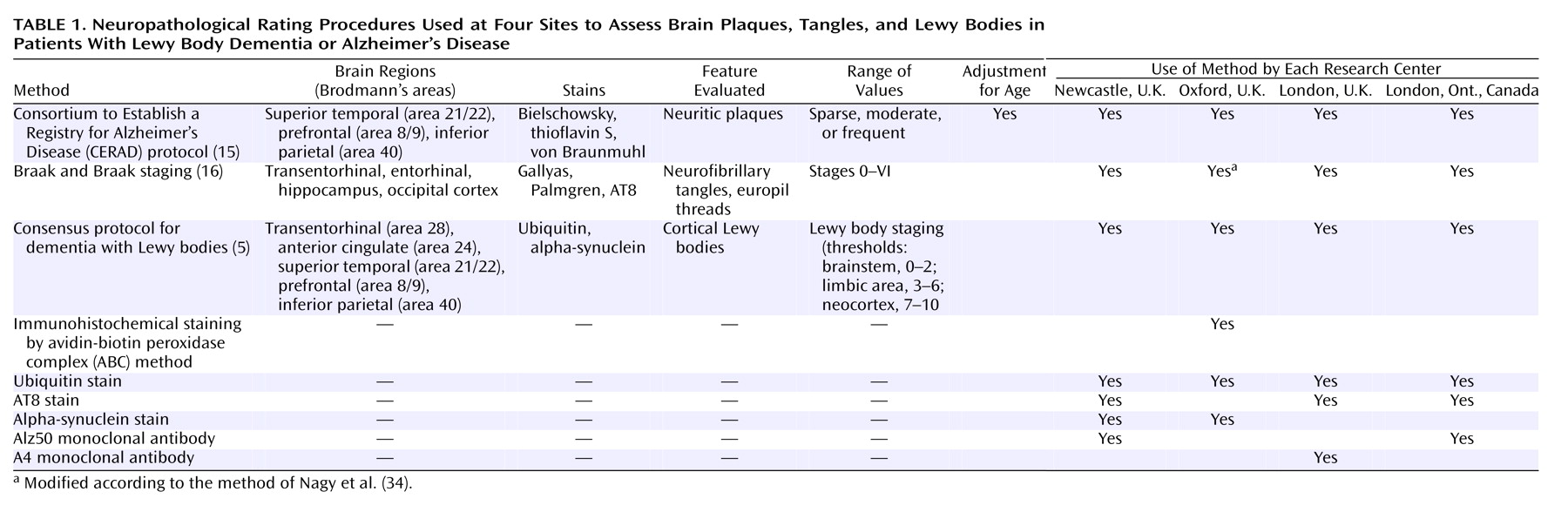
The neuropathological methods and the staining procedures used at each center are summarized in
Table 1.
An additional 90 patients with neuropathologically confirmed Alzheimer’s disease from the three U.K. centers, who had been assessed prospectively during life with the same standardized procedures, were included as a comparison group. For the patients with Lewy body dementia, the Braak stages, CERAD stages, and Lewy body dementia stages were correlated in independent analyses with key symptoms by using the chi-square test. The numbers of patients with Lewy body dementia and with Alzheimer’s disease who experienced the symptoms of interest at presentation and the numbers who had them persistently (≥6 months) during the course of the dementia were compared by using chi-square tests. Correlations between different pathologies were undertaken by means of Spearman’s rank correlation. The Statistical Package for the Social Sciences was used for all evaluations
(35).
Results
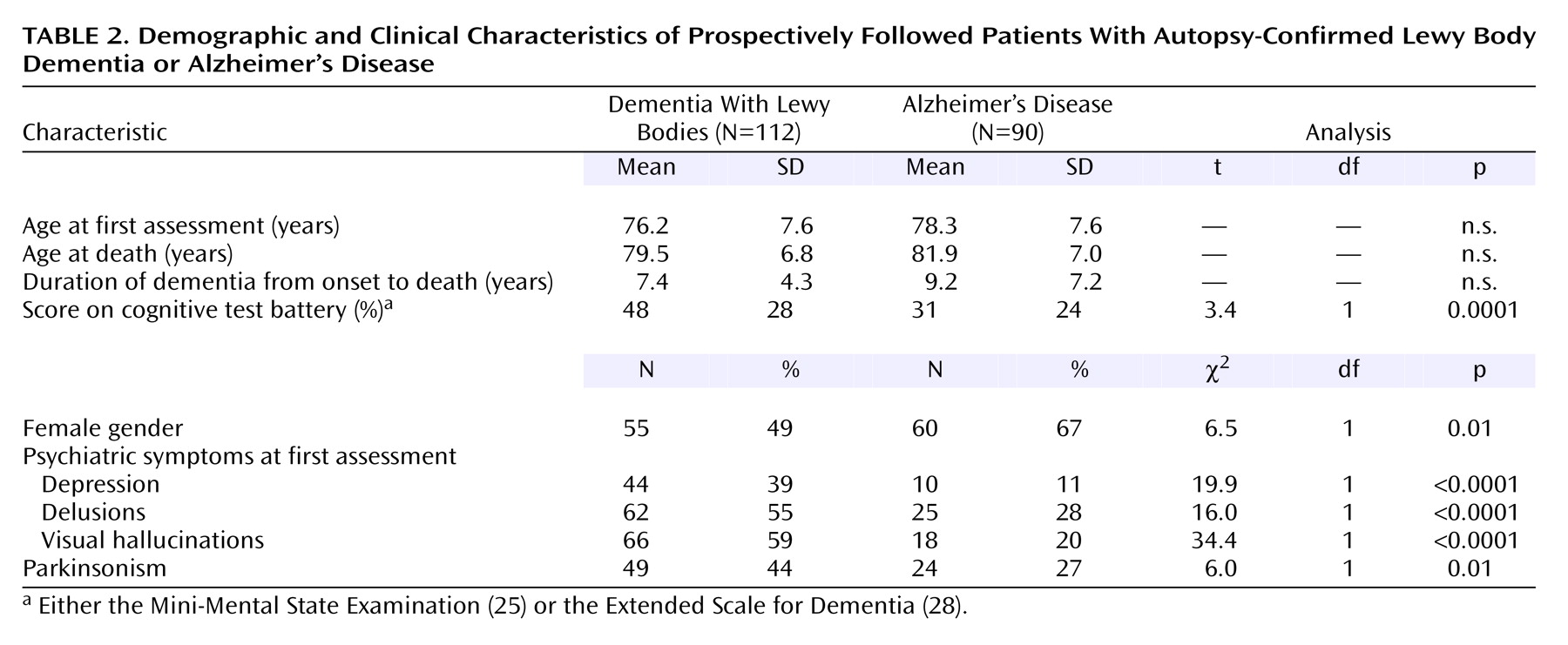
Nearly half of the patients who had dementia with Lewy bodies were female. Their mean age and duration of dementia are presented in
Table 2. The median duration between the baseline assessment and death was 2 years, and the median number of clinical assessments for the patients with Lewy body dementia was three per participant, although 23 (21%) of the patients died before any follow-up evaluations were completed. There were high frequencies of visual hallucinations, delusions, and major depression among the patients with Lewy body dementia (
Table 2).
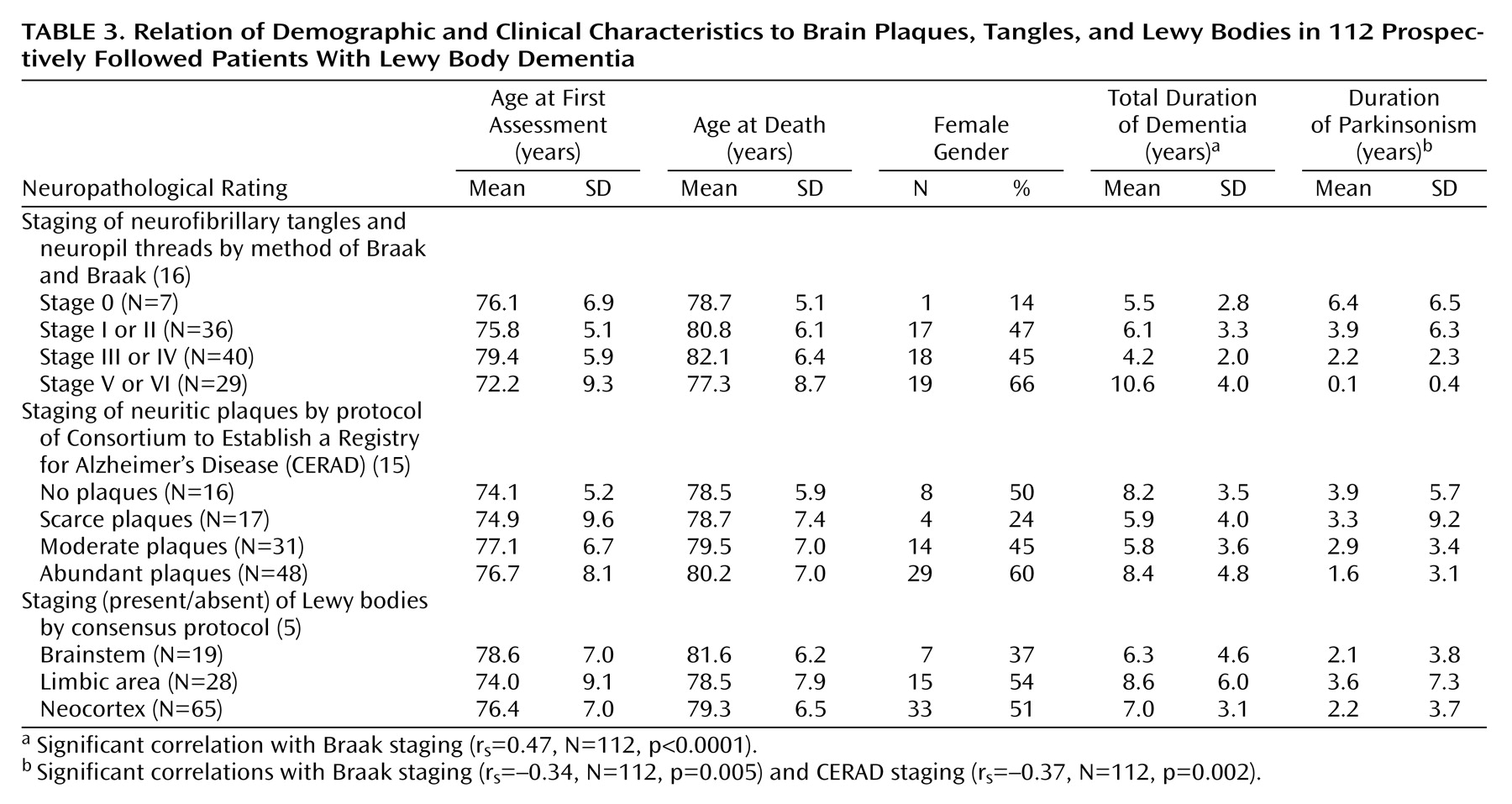
Braak staging was significantly correlated with dementia duration (
Table 3), but there was no association between the number of years of dementia and Lewy body staging. As anticipated, Braak and CERAD staging were significantly correlated (r
s=0.40, N=112, p<0.0001). The severity of Lewy body pathology was independent of plaques (CERAD staging: r
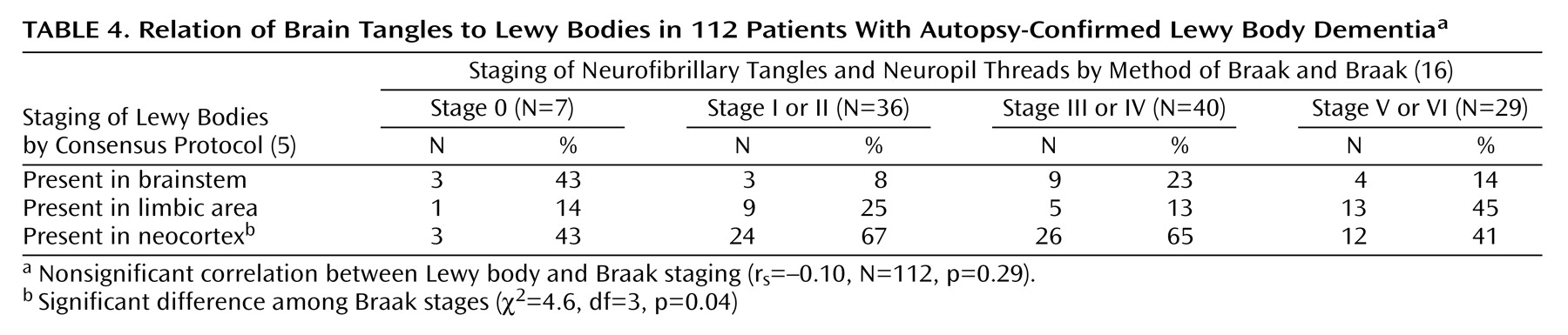
s=0.12, N=112, p=0.19), but there was a significantly lower proportion of patients with neocortical Lewy bodies among those with Braak stage V or VI tangle pathology (
Table 4).
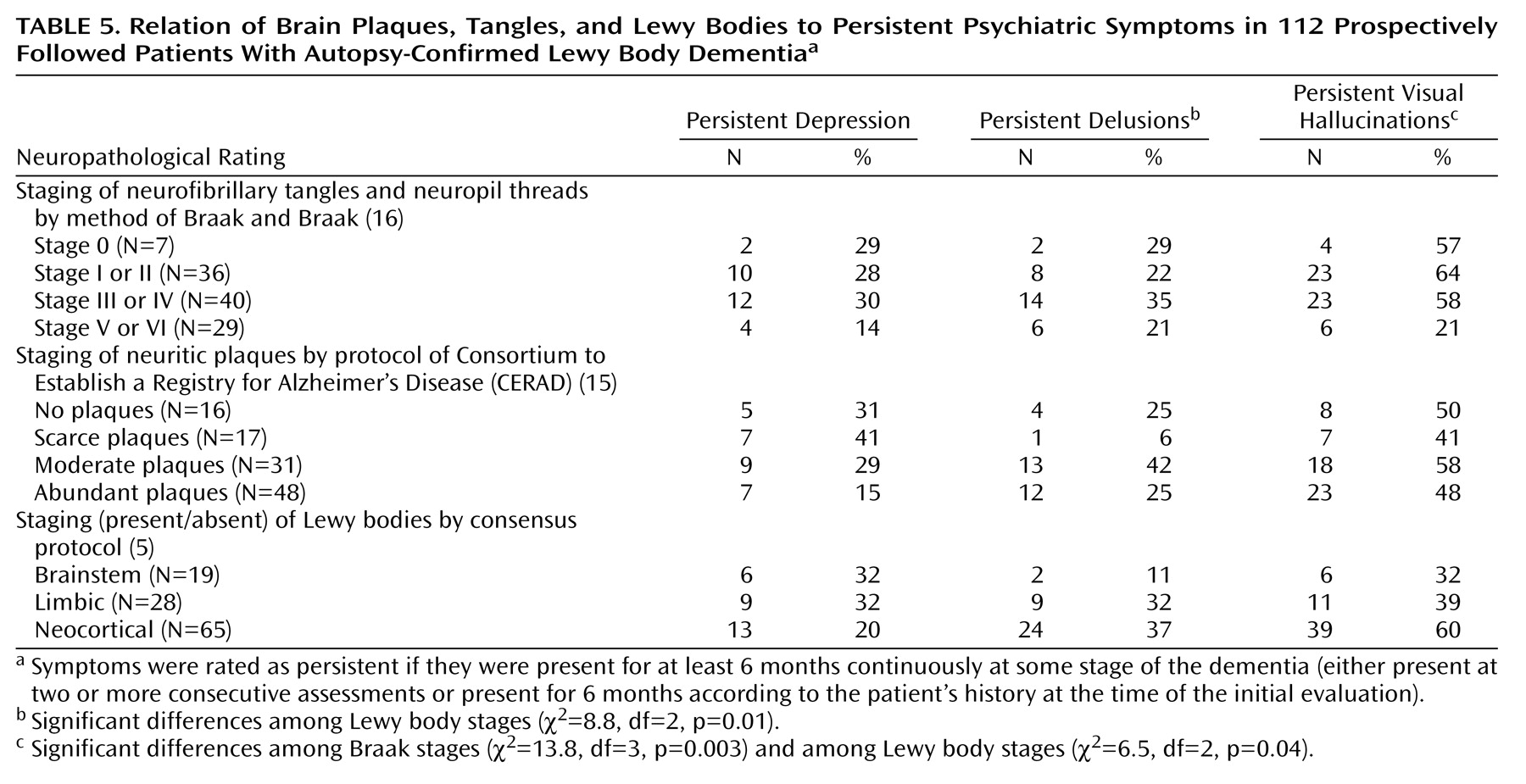
Within the group who had Lewy body dementia there was a significant association between lower Braak stage and higher frequency of persistent visual hallucinations (
Table 5). There was no association between the CERAD staging and any of the psychiatric symptoms. Significant associations between higher Lewy body staging and both persistent delusions and persistent visual hallucinations were identified. Persistent visual hallucinations were most frequent in patients with neocortical Lewy bodies.
The characteristics of the groups with Lewy body dementia and Alzheimer’s disease are shown in
Table 2. Compared to those with Alzheimer’s disease, the patients with Lewy body dementia had significantly higher frequencies of depression, delusions, visual hallucinations, and parkinsonism at the beginning of the study. Longitudinal data were available for 77 (86%) of the Alzheimer’s disease patients, of whom 14 (18%) had persistent visual hallucinations, 24 (31%) had persistent delusions, and 14 (18%) had persistent depression. In comparison with the patients who had Lewy body dementia, the Alzheimer’s disease patients were significantly less likely to have persistent visual hallucinations (χ
2=19.4, df=1, p<0.0001) but not delusions (χ
2=0.48, df=1, p=0.49) or depression (χ
2=0.73, df=1, p=0.39). Patients with Lewy body dementia who had a higher Braak stage (V or VI) had a frequency of persistent visual hallucinations (21%) that was more similar to that of the patients with Alzheimer’s disease (20%) than the frequency for patients with Lewy body dementia who had a Braak stage of 0–II (
Table 2,
Table 5).
Discussion
This study of patients with autopsy-confirmed Lewy body dementia elucidates the relationship of pathology to clinical features in dementia with Lewy bodies. There were two main findings. First, a low tangle count is the main correlate of persistent visual hallucinations in dementia with Lewy bodies, but the presence of neocortical Lewy bodies also increases the frequency of both visual hallucinations and delusions. Second, patients who have Lewy body dementia with Braak stage V or VI tangle pathology have a symptom profile more similar to that of Alzheimer’s disease patients than do other patients with Lewy body dementia, and they also have significantly fewer neocortical Lewy bodies. This indicates that patients with Lewy body dementia with higher tangle counts and fewer neocortical Lewy bodies are a discrete subgroup with different clinical and neuropathological characteristics. This series confirms that patients with Lewy body dementia have high frequencies of visual hallucinations (59%), delusions (55%), and depression (39%), each of which was present significantly more frequently than in Alzheimer’s disease patients, and also that visual hallucinations (present for 6 months or more in 50% of the patients with Lewy body dementia), but not other psychiatric features, are significantly more persistent.
Patients with Lewy body dementia who had Braak stage V or VI tangle pathology had a frequency of persistent visual hallucinations more similar to that of the Alzheimer’s disease patients than to that of the patients with Lewy body dementia who had Braak stage 0–IV pathology. This is important for diagnosis, as a lower proportion of these patients meet operationalized clinical criteria for dementia with Lewy bodies. Taken together with the lower proportion of patients with neocortical Lewy bodies, this finding hence provides some justification for considering this group as having a “Lewy body variant of Alzheimer’s disease.” This concept has been described previously in relation to plaque pathology
(1) but, on the basis of the present data, should be focused on patients with Lewy body dementia who have severe tangle pathology. It will be important to determine whether “Lewy body variant” patients experience severe neuroleptic sensitivity reactions and how responsive their neuropsychiatric symptoms are to cholinesterase inhibitors.
Visual hallucinations and delusions in patients with Lewy body dementia have different pathological substrates, a finding that is consistent with results from previous neurochemical studies
(14), and the neuropathological associations of psychosis are different in dementia with Lewy bodies and Alzheimer’s disease. In Alzheimer’s disease patients there is a significant positive association between the presence of neurofibrillary tangles in the neocortex and the occurrence of psychotic symptoms, defined as either visual hallucinations or delusions
(36); this relationship is the opposite of the inverse association between visual hallucinations and neurofibrillary tangle staging in the current study. This difference may be due to the fact that the patients with Lewy body dementia had more pronounced cholinergic deficits in the medial temporal lobe than did the patients with Alzheimer’s disease, despite a lower tangle burden
(14,
37). The different underlying bases of visual hallucinations and delusions and the different neuropathological substrates of psychotic symptoms in dementia with Lewy bodies and Alzheimer’s disease are crucial for developing a meaningful classification of psychiatric syndromes in dementia patients, for designing clinical trials, and for developing rational treatment approaches. The results of treatment studies focusing on psychiatric symptoms in dementia with Lewy bodies hence do not enable us to predict the responsiveness of these symptoms to the same treatments in Alzheimer’s disease patients.
The presence of persistent visual hallucinations was significantly inversely associated with Braak stage. More widespread Lewy bodies were also associated with the persistence of visual hallucinations. Previous work indicates that visual hallucinations in dementia with Lewy bodies (and also Parkinson’s disease) are related to the severity of cholinergic deficits
(14) and probably also to the extent of cortical Lewy body pathology
(11), particularly in the temporal cortex
(24). The present findings are consistent with those of previous studies but also emphasize the importance of less severe tangles. Delusions were not associated with plaque or tangle staging in our study, although persistent delusions were associated with Lewy body distribution. While these findings have important implications for the classification of cases of dementia with Lewy bodies and for understanding differences in the neuropathological basis of psychiatric symptoms in Lewy body dementia and Alzheimer’s disease, this is only the first step. Further work needs to evaluate in detail the pattern of associations between plaque, tangle, and Lewy body pathology in specific regions of interest. Important smaller studies have been reported; for example, Harding et al.
(24) reported an association between visual hallucinations and the density of Lewy bodies in key temporal lobe regions in 29 patients with Lewy body dementia. Building on this work in large systematic studies will be important to develop a more comprehensive understanding of the neuropathological substrates of key symptoms in dementia with Lewy bodies.
Although frequent, depression in patients with Lewy body dementia was not associated with plaque, tangle, or Lewy body pathology. Consistent with this finding was the association with serotonergic measures in a previous neurochemical study
(38).
Lewy bodies were less prevalent in patients with high Braak staging, consistent with earlier evidence that there may be a degree of mutual exclusion between Lewy body and tau pathology. According to Arima et al.
(39), the presence of neurofibrillary tangles or tau-epitopes within neurons containing Lewy bodies is rare, although in a more recent study
(40) using tau and alpha-synuclein immunohistochemistry the coexistence of neurofibrillary tangles and Lewy bodies in the same neurons in the subiculum was identified. Both of these pathological studies suggested that alpha-synuclein stimulates the accumulation of phosphorylated tau in terminal axons.
To our knowledge, this is the largest comparison of dementia with Lewy bodies and Alzheimer’s disease. At each center the patients were diagnosed at autopsy but had been prospectively assessed during life with standardized clinical evaluations. We determined the correlations of initial symptoms and persistent symptoms (of 6 months’ duration) over the course of the illness to neuropathological features at death. The first patients to come to autopsy in longitudinal series usually die of concurrent causes in the mild to moderate stages of the disease. As a result, the median duration between first assessment and death was only 2 years. The longitudinal design has particular advantages for the evaluation of associations with persistent psychosis.
Assessments were completed with validated staging systems, although the detailed methodological procedures differed between the individual centers. The biggest potential disadvantage is the different staining procedures used for the identification of Lewy bodies. While this may have been a problem for the detailed quantification of Lewy body density, it is unlikely to have made a substantial difference to the standard staging procedure. For example, in a comparative study, alpha-synuclein staining improved the identification of Lewy bodies by only 5% over other methods
(18).
In conclusion, in patients with Lewy body dementia, the major neuropathological characteristic associated with persistent visual hallucinations is the absence of severe tangle pathology, indicating a different neuropathological basis from that previously described for psychotic symptoms in patients with Alzheimer’s disease. An important implication is that treatment response is also likely to be different, and results of clinical trials cannot easily be generalized from one patient group to the other.