The selective serotonin reuptake inhibitors (SSRIs) were first introduced in the United States in 1988 and remain the antidepressants of first choice. Because the serotonin transporter (5-HTT) is believed to be the primary therapeutic target of SSRIs, genetic polymorphisms of the 5-HTT have been investigated in relationship to the antidepressant response to SSRIs. Smeraldi et al.
(1) reported that the presence of the L allele of the 5-HTT gene-linked polymorphic region (5-HTTLPR) conferred a superior therapeutic response to fluvoxamine. Several subsequent studies in Europe and the United States revealed similar results
(2–
4). In contrast, in a Korean study, Kim et al.
(5) reported that the S/S homozygous allele pairs were more frequently associated with a therapeutic response to fluoxetine or paroxetine than other allele combinations. Concordant with this result is our finding that the S allele occurs more frequently in fluvoxamine-responsive Japanese patients than in nonresponsive patients
(6). It is unclear what mechanism may underlie ethnic differences in antidepressant response. Several other serotonergic system genetic polymorphisms have also been scrutinized in relationship to the antidepressant response to SSRIs
(7–
11), but a consistent pattern of results has not emerged.
Recently, dual serotonin/norepinephrine reuptake inhibitors (SNRIs) have emerged as particularly effective antidepressants. Unfortunately, there are few validated predictors of antidepressant response, except for past and family history of response
(12). Prediction of antidepressant response to SNRIs would represent an important advance in the field. Our previous study
(13) revealed that there is no significant relationship between plasma concentrations of milnacipran, an SNRI, and the antidepressant response.
To our knowledge, there have been no reports published to date on the relationship between genetic polymorphisms and antidepressant response to SNRIs. Because SNRIs act not only on the 5-HTT but also on the norepinephrine transporter (NET), the effects of polymorphisms of both transporters are of interest. In the current study, we examined the effect of NET and 5-HTT polymorphisms on the antidepressant response to milnacipran. In addition, we also investigated a genetic polymorphism of the 5-HT2A receptor.
Method
Subjects and Treatment
Ninety-six Japanese patients who fulfilled DSM-IV criteria for a diagnosis of major depressive disorder and whose scores on the Montgomery-Åsberg Depression Rating Scale
(14) were 21 or higher were included in this study. Patients with other axis I disorders (including dementia, substance abuse, dysthymia, panic disorder, obsessive-compulsive disorder, and generalized anxiety disorder) and those with axis II disorders determined by clinical interview were excluded. Patients with a past history of childhood disorders were also excluded, as were patients with severe nonpsychiatric medical disorders.
The patients were 20–69 years of age and had been free of psychotropic drugs for at least 14 days before entry into the study. After complete description of the study to the subjects, written informed consent was obtained.
Milnacipran was administered twice daily (after dinner and at bedtime in the same dose) for 6 weeks. The initial total dose was 50 mg/day, and after 1 week it was increased to 100 mg/day. Patients with insomnia were prescribed brotizolam, 0.25 or 0.5 mg, a benzodiazepine sedative hypnotic, at bedtime. No other psychotropic drugs were permitted during the study. Of 96 enrolled patients, 10 did not complete the study—five patients because of side effects, one patient because of severe insomnia, and four patients without explanation. Of the 86 patients who completed the 6-week study, six patients were excluded from the current analysis because plasma samples revealed very low milnacipran concentrations, indicative of poor compliance.
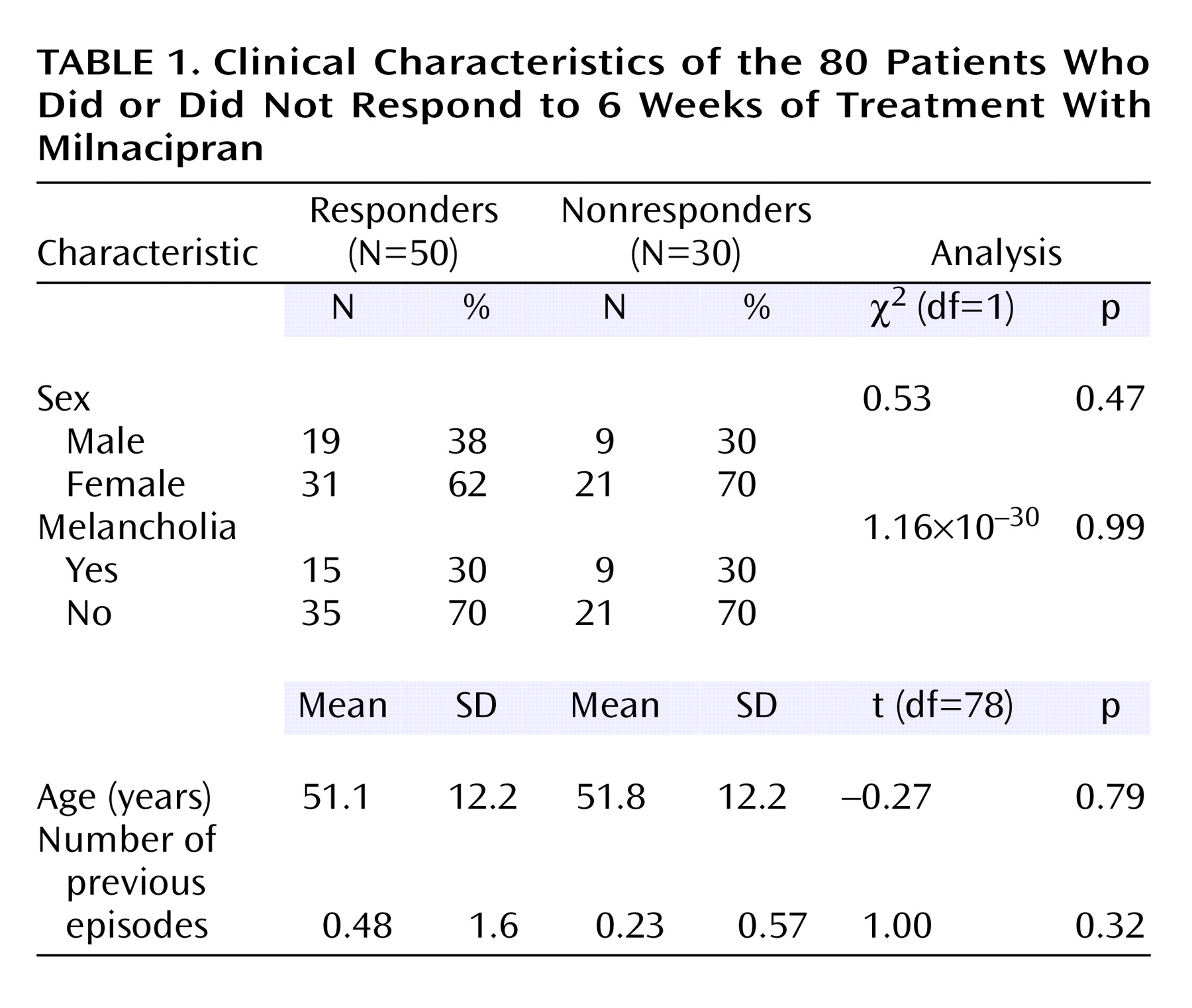
Patients who completed the study included 52 women and 28 men, 49 outpatients and 31 inpatients, 25–69 years of age (mean age=51.4, SD=12.2). The clinical characteristics of the patients are shown in
Table 1. The number of previous depressive episodes among these patients was very low. Indeed, 64 (80%) of the 80 patients were experiencing their first episode. None of the patients had childhood-onset depression.
Data Collection
Depression symptom severity was assessed with the use of the Montgomery-Åsberg Depression Rating Scale. Assessments were conducted at baseline and at 1, 2, 4, and 6 weeks after initiation of antidepressant treatment. A single rater conducted each of the ratings for each patient. A clinical response was defined as a 50% or greater decrease in the baseline Montgomery-Åsberg Depression Rating Scale score. Clinical remission was defined as a final Montgomery-Åsberg Depression Rating Scale score less than 10. Collection of blood samples was performed 12 hours after drug administration at bedtime, 4 weeks after initiation of milnacipran treatment.
Genotyping
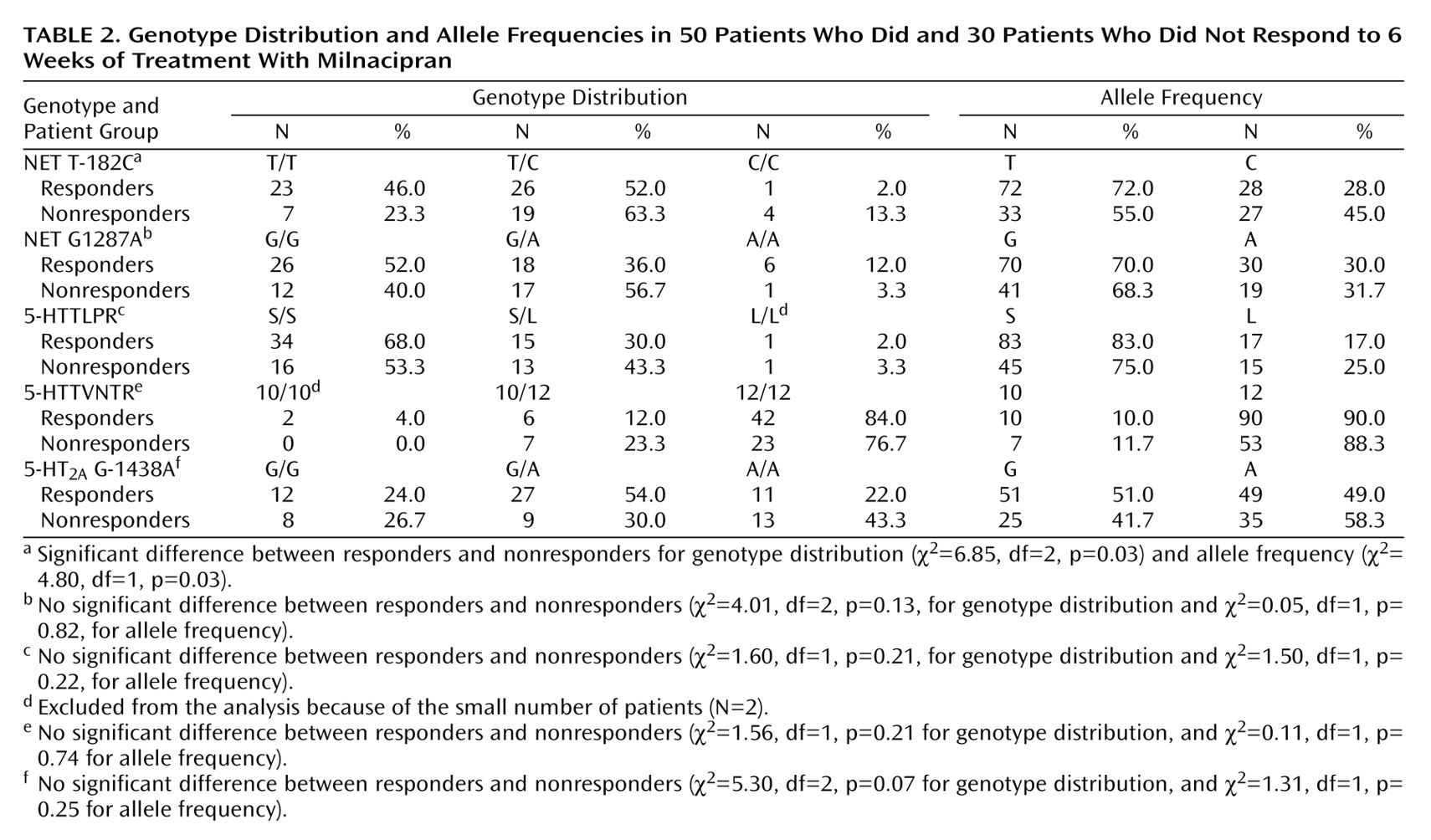
NET polymorphisms
T-182C polymorphism in the promoter region (NET T-182C) was determined by a modification of the method of Zill et al.
(15). G1287A polymorphism in exon 9 (NET G1287A) was determined by the method of Jönsson et al.
(16).
5-HTT polymorphisms
5-HTTLPR, a variable number of tandem repeats in the second intron of the 5-HTT gene (5-HTTVNTR), and a G-1438A polymorphism in the promoter region of the 5-HT
2A receptor gene (5-HT
2A G-1438A) were determined by using the method of polymerase chain reaction. The method is described in detail in previous publications
(6,
9,
10).
Quantification of Plasma Milnacipran Concentration
Plasma concentrations of milnacipran were measured by using high performance liquid chromatography. Details of the method have been described previously
(13). Milnacipran was measured in duplicate samples; the mean of the two samples is presented.
Genotyping and measurement of plasma milnacipran concentrations were performed by laboratory personnel blind to the identity and clinical response of the patients. Moreover, the clinicians were unaware of the genotyping results and plasma milnacipran concentrations of each patient.
Statistical Analysis
Differences in patient characteristics were analyzed with the use of the unpaired t test or chi-square test where appropriate. Differences in the time course of Montgomery-Åsberg Depression Rating Scale scores were examined with the use of repeated-measures analysis of variance (ANOVA). Genotype distribution and allele frequencies were analyzed with the use of the chi-square test. Plasma concentrations of milnacipran were analyzed with the use of one-way factorial ANOVA in each genotype group; an unpaired t test was then used to analyze differences between groups who were or were not responsive to milnacipran. Power analysis was performed with the use of G⋅Power
(17). All tests were two-tailed; alpha was set at 0.05.
Discussion
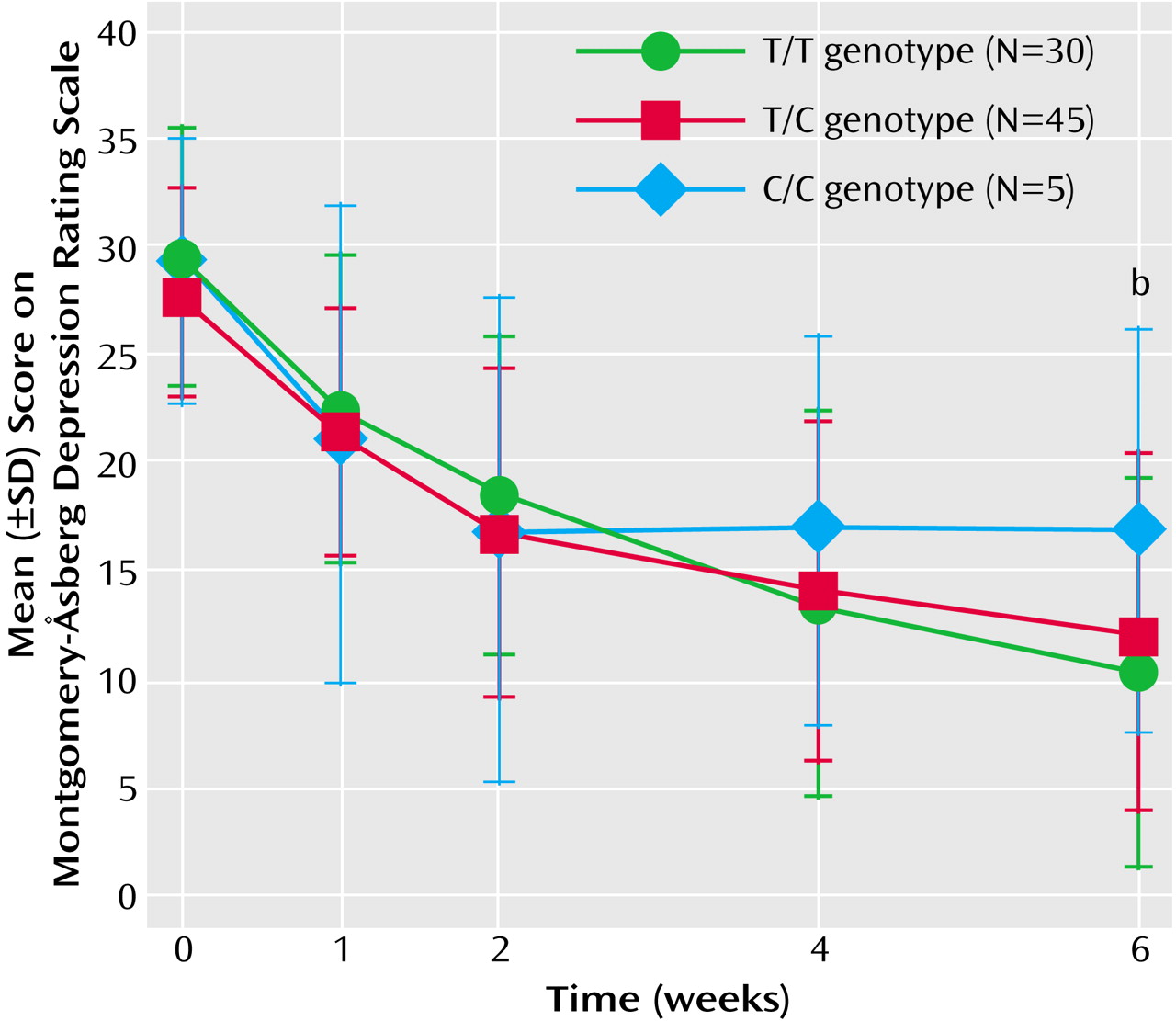
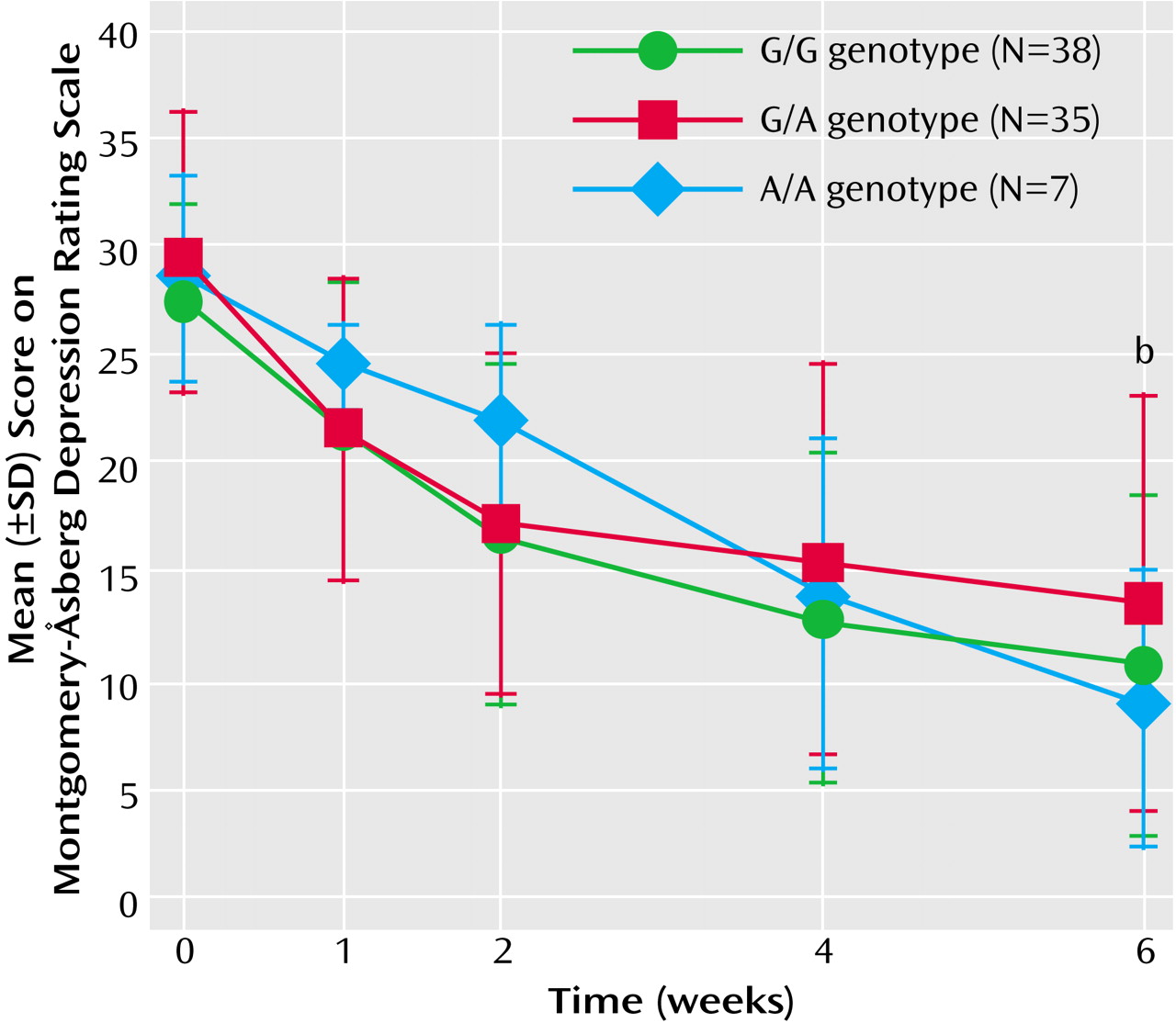
The present study revealed that NET but not 5-HTT polymorphisms affected the antidepressant response to milnacipran, an SNRI. The presence of the T allele of the NET T-182C polymorphism was associated with a greater therapeutic response, and the presence of the A/A genotype of the NET G1287A polymorphism was associated with a slower onset of therapeutic response.
Several investigators have studied the relationship between NET genetic polymorphisms and susceptibility to psychiatric disorders, including major depression, bipolar disorder, schizophrenia, and alcohol dependence
(15,
18–20), without observing major findings. However, our findings in the present study suggest that it is possible to predict the antidepressant response to an SNRI with the use of NET polymorphisms. It is unclear why the 5-HTTLPR polymorphism does not predict the antidepressant response to milnacipran. As noted, controversy exists in this area concerning SSRI response.
The NET G1287A polymorphism was reported to be a silent mutation
(21). However, Jönsson et al.
(16) reported that this polymorphism was associated with the CSF concentration of 3-methoxy-4-hydroxyphenylglycol (MHPG), a major norepinephrine metabolite. They reported that CSF MHPG concentrations were higher in the G/G genotype than in the G/A and A/A genotypes. The NET G1287A polymorphism may be in linkage disequilibrium with an as-yet unidentified functional polymorphism. Higher concentrations of MHPG have been postulated to be attributable to more active reuptake of norepinephrine. Because patients with the A/A genotype have less active reuptake of norepinephrine, the effect of reuptake inhibition might occur more slowly.
The NET T-182C polymorphism was first reported by Zill et al.
(15), and its functional consequences remain obscure. Such functional information would aid in interpretation of the findings obtained in this study. Further studies are needed to analyze its potential effects on NET expression.
Collier et al.
(22) reported that the transcriptional activity of the L allele of the 5-HTTLPR polymorphism was more than twice as high as that of the S allele in transformed lymphoblast cell lines. However, using positron emission tomography, Shioe et al.
(23) recently reported that 5-HTT binding in vivo was not significantly different among the 5-HTTLPR genotypes. Lovejoy et al.
(24) reported that individual repeat elements within the 5-HTTVNTR domain differed in their enhancer activity in an embryonic stem cell model. These elements may be active in response to specific stimuli, but their functional effect in vivo has not been reported. Turecki et al.
(25) reported that the 5-HT
2A G-1438A polymorphism affected 5-HT
2A receptor binding in postmortem brain samples, but Bray et al.
(26) reported that the 5-HT
2A G-1438A variant did not exert a significant effect on 5-HT
2A receptor mRNA expression in postmortem brain tissue. We noted no consistent effect of serotonergic genetic polymorphisms in this study.
One major limitation of this study is the relatively small number of subjects and the fact that the endpoint treatment differences were relatively small. A second limitation is the short duration of treatment. The washout period in this study was 2 weeks. Most of the patients in this study were experiencing their first episode of depression and were medication-free before entry into the study. Several patients had previously been treated with SSRIs, but the only available SSRIs in Japan are fluvoxamine and paroxetine. Fluoxetine, a very long half-life SSRI, is not available in Japan.
Patients with comorbid axis I or axis II disorders were excluded to ensure a relatively homogeneous sample. The relatively high rate of substance abuse in the United States (8.3% of the population 12 years old or older was reported to have used any illicit drug during the past month
[27]) may suggest that it is unusual that we could select patients with no comorbid substance abuse. However, in Japan, only 0.1% of the population 15 years old or older had used illicit substances during the past year
(28). The low prevalence rate of substance abuse in Japan renders it relatively easy to select patients with major depressive disorder who do not have substance abuse.
The results of this study should be confirmed outside of Japan, because ethnic differences have been reported in studies seeking to determine the influence of the 5-HTTLPR polymorphism on the antidepressant response to SSRIs
(29).