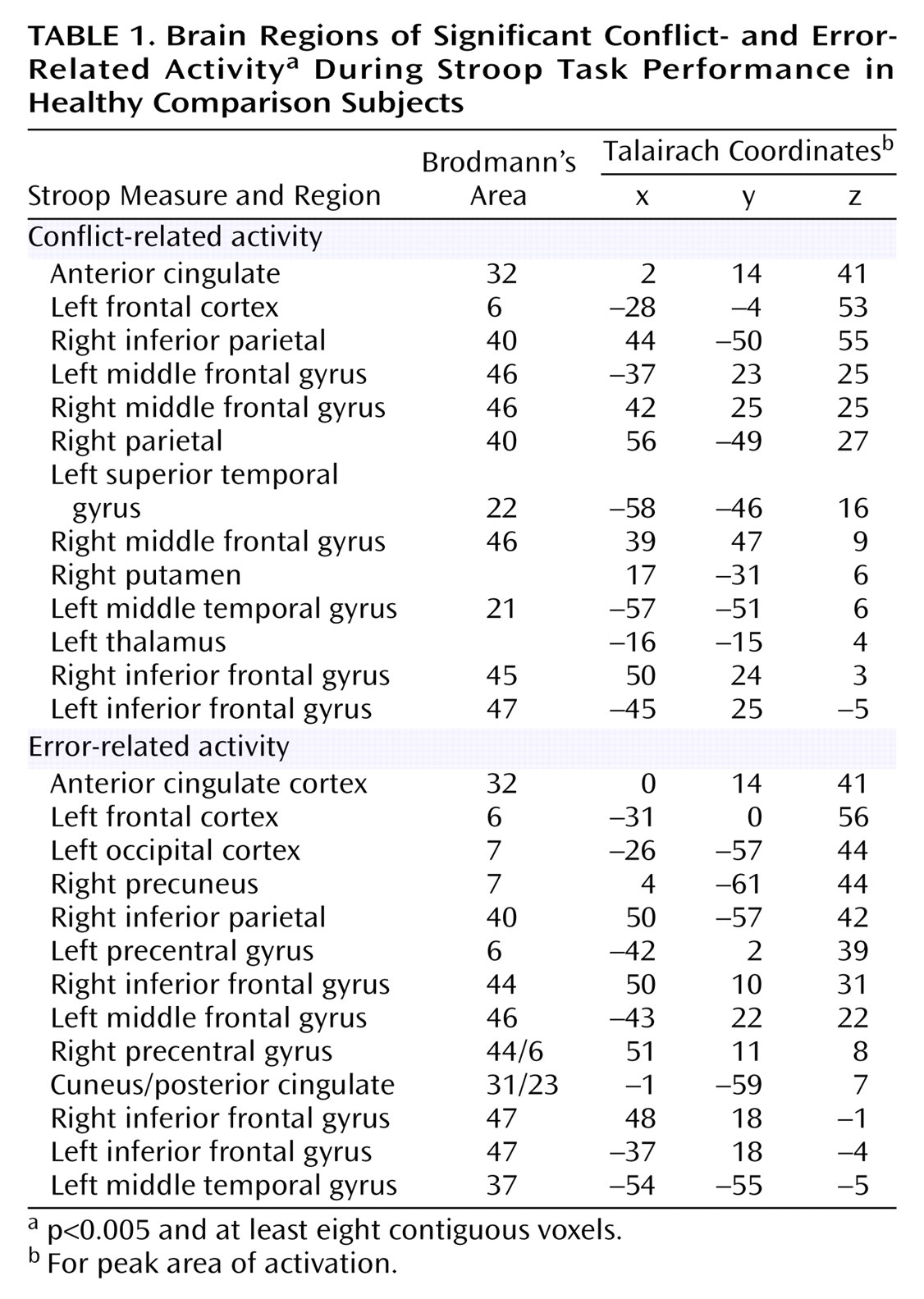
People with schizophrenia exhibit deficits in cognitive control
(1). An important brain region involved in cognitive control is the anterior cingulate cortex
(2). The goal of the current study was to test whether anterior cingulate cortex dysfunction in people with schizophrenia can be attributed to impairment in a specific cognitive function, the monitoring of response conflict.
Schizophrenia patients have frequently been reported to exhibit reduced anterior cingulate cortex activity at rest
(3), during the performance of many different types of cognitive tasks
(4–
6), and during the commission of errors
(7). In event-related potential studies, people with schizophrenia exhibit a reduced error-related negativity
(8–
11), an event-related potential component associated with the commission of errors with a source consistent with the anterior cingulate cortex
(12,
13).
One theory of anterior cingulate cortex function is that it is involved in monitoring response conflict (i.e., the simultaneous activation of two competing responses)
(2). For example, in the Stroop task, conflict occurs between color naming and word reading (e.g., the word “RED” printed in green ink). Response conflict occurs in many different tasks (e.g., underdetermined responding) and might explain why anterior cingulate cortex activity has been observed in a wide range of functional brain imaging studies
(14).
According to conflict theory, the occurrence of response conflict elicits a signal for greater cognitive control, resulting in recruitment of brain regions to overcome conflict through increased control
(2). Moreover, conflict theory posits that anterior cingulate cortex activity during errors is also the result of response conflict
(15), since after an error ongoing stimulus evaluation leads to concurrent activation of both the correct and the just executed incorrect response. Hence, conflict theory predicts that the same area of the anterior cingulate cortex should be active during both conflict occurrence and error commissions (errors being a high conflict state), and that both signals should be associated with adjustments in control
(2,
15). An alternative view of anterior cingulate cortex function is that it is directly involved in attentional selection. However, previous research suggests that the anterior cingulate cortex is involved in detecting conflict, whereas the prefrontal cortex is involved in selective attention
(16,
17).
On the basis of conflict theory, we predicted that impaired anterior cingulate cortex activity in schizophrenia would be related to impaired conflict monitoring. If true, people with schizophrenia should exhibit reduced anterior cingulate cortex activity in the same area of the anterior cingulate cortex during both high-conflict correct trials and during commission of errors. However, concurrently decreased anterior cingulate cortex activity during both conflict and error trials has not been examined in the same study. Moreover, conflict theory predicts reduced behavioral adjustments after the occurrence of both conflict and errors in schizophrenia. Previous research has yet to examine whether people with schizophrenia exhibit reduced post-conflict behavioral adjustments and has been inconclusive regarding whether people with schizophrenia show reduced post-error adjustments, with two studies reporting reduced
(7,
8) and two studies reporting intact
(10,
11) adjustments.
Results
Behavioral Results
A two (trial type: congruent versus incongruent) by two (group: patient versus comparison) analysis of variance found a large and significant Stroop behavioral effect (congruent reaction time < incongruent reaction time: F=15.95, df=1, 24, p<0.001). Although not significant, schizophrenia subjects tended to have a larger Stroop effect (congruent mean=609.9 msec [SD=215.1]; incongruent mean=807.8 msec [SD=449.6]) than did the comparison subjects (congruent mean=476.9 msec [SD=92.0]; incongruent mean=567.3 msec, [SD=150.9]) (F=2.21, df=1, 24, p=0.15). There was also a tendency for schizophrenia subjects to have slower reaction times in general than comparison subjects (F=3.69, df=1, 24, p<0.07).
As expected, comparison subjects exhibited robust, significant post-conflict adjustment (32 msec) (t=2.70, df=12, p<0.05) and post-error slowing (26 msec) (t=2.56, df=12, p<0.05). In contrast, people with schizophrenia exhibited neither significant post-conflict adjustment (7 msec) (t=0.30, df=12, p>0.70) nor significant post-error slowing (4 msec) (t=0.19, df=11, p>0.80). In addition, there was an overall significantly lower total behavioral adjustment score in patients (t=2.12, df=24, p<0.05) (specific between-group comparison for post-conflict adjustment: t=1.63, df=24, p=0.17; for specific post-error slowing: t=1.63, df=23, p=0.12).
fMRI Results
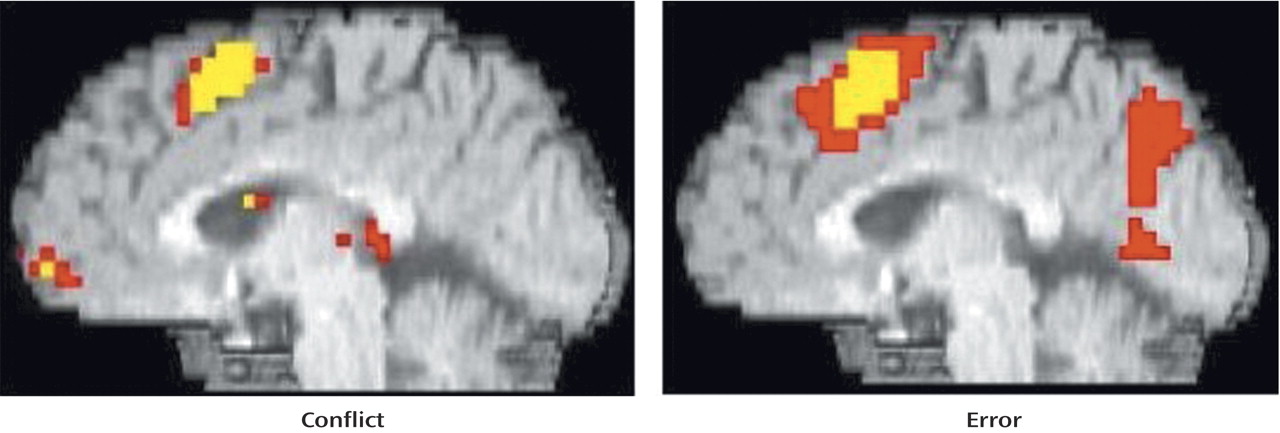
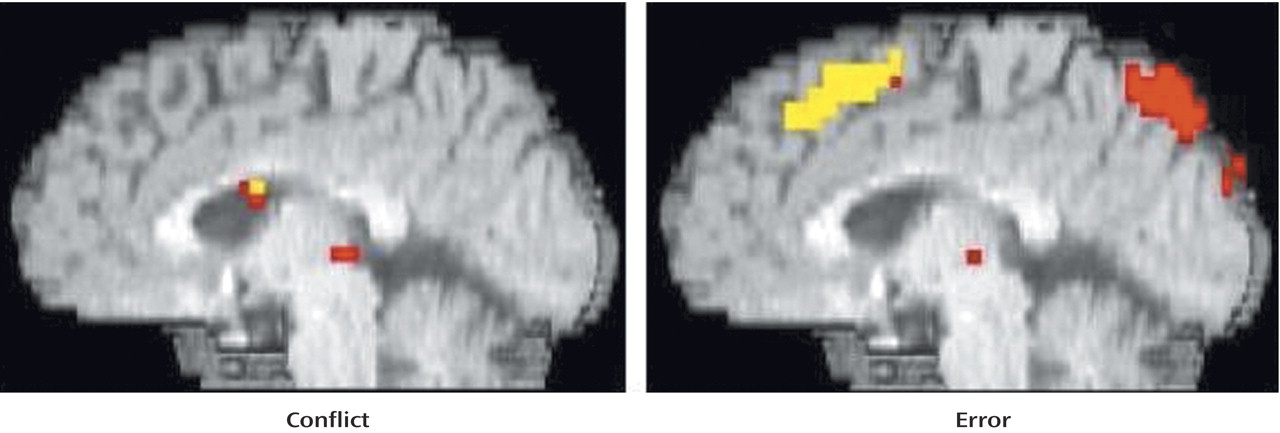
As can be seen in
Figure 1 and
Table 1, comparison subjects exhibited robust conflict- and error-related activity in the anterior cingulate cortex (extending into the supplementary motor area), with conflict and error regions of activity largely overlapping. In fact, the conflict region fell entirely within the error region, with the error region being more extensive in every dimension (extent of difference in y dimension=3 mm; extent of inferior difference in z dimension=4 mm). Using the anterior cingulate cortex region of conflict-related activity as a region of interest, we found a significant amount of error-related activity in that same region. Similarly, we found a significant amount of conflict-related activity in the anterior cingulate cortex region of error-related activity.
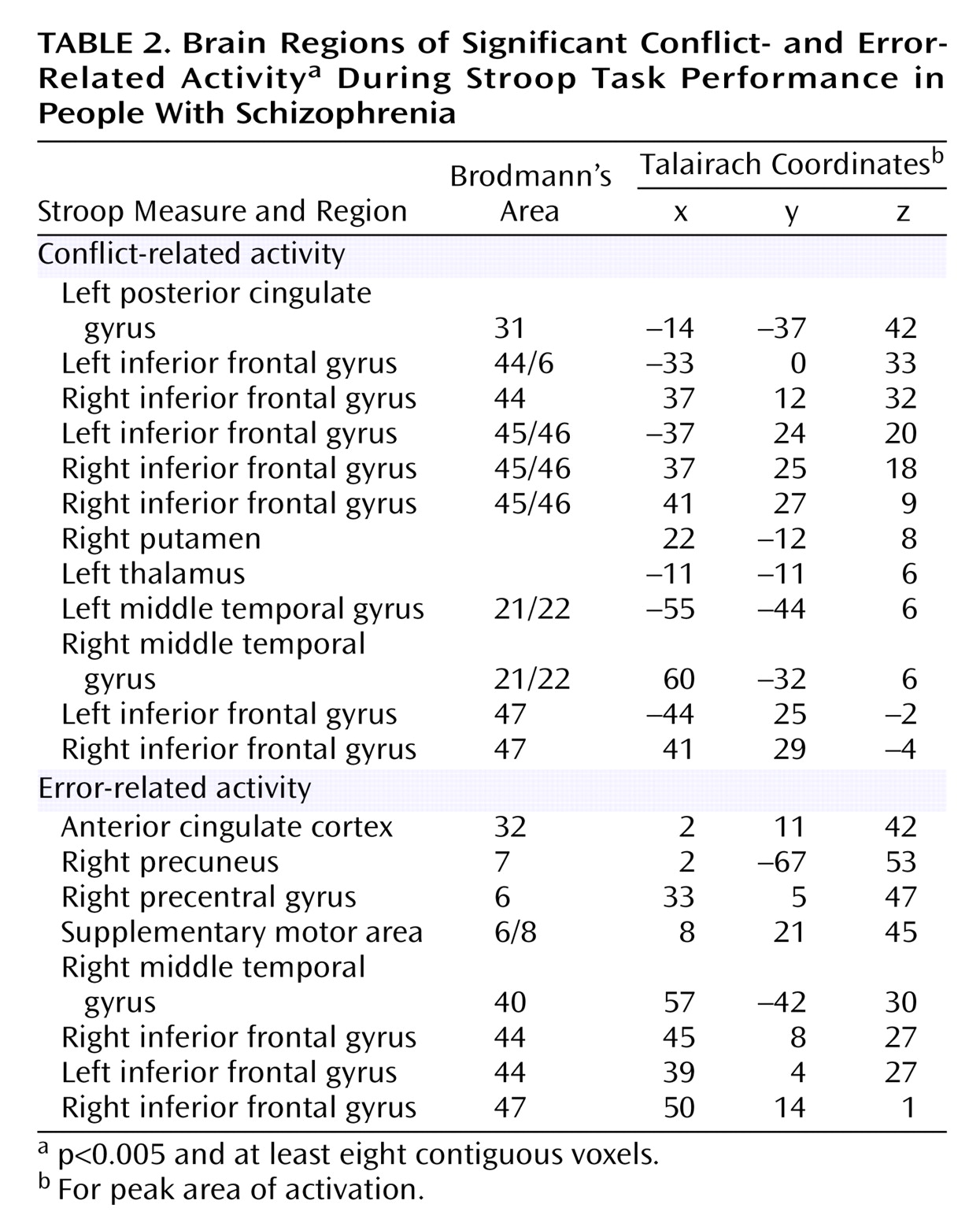
As can be seen in
Figure 2 and
Table 2, people with schizophrenia also exhibited significant error-related activity in the anterior cingulate cortex, although most of their error activity occurred superiorly on the medial wall. In contrast, people with schizophrenia did not exhibit significant conflict-related anterior cingulate cortex activity. Even after lowering the threshold to p<0.05, people with schizophrenia did not exhibit significant conflict-related activity in the anterior cingulate cortex. As can be seen in
Table 2, people with schizophrenia did exhibit robust and significant conflict-related activity in other regions.
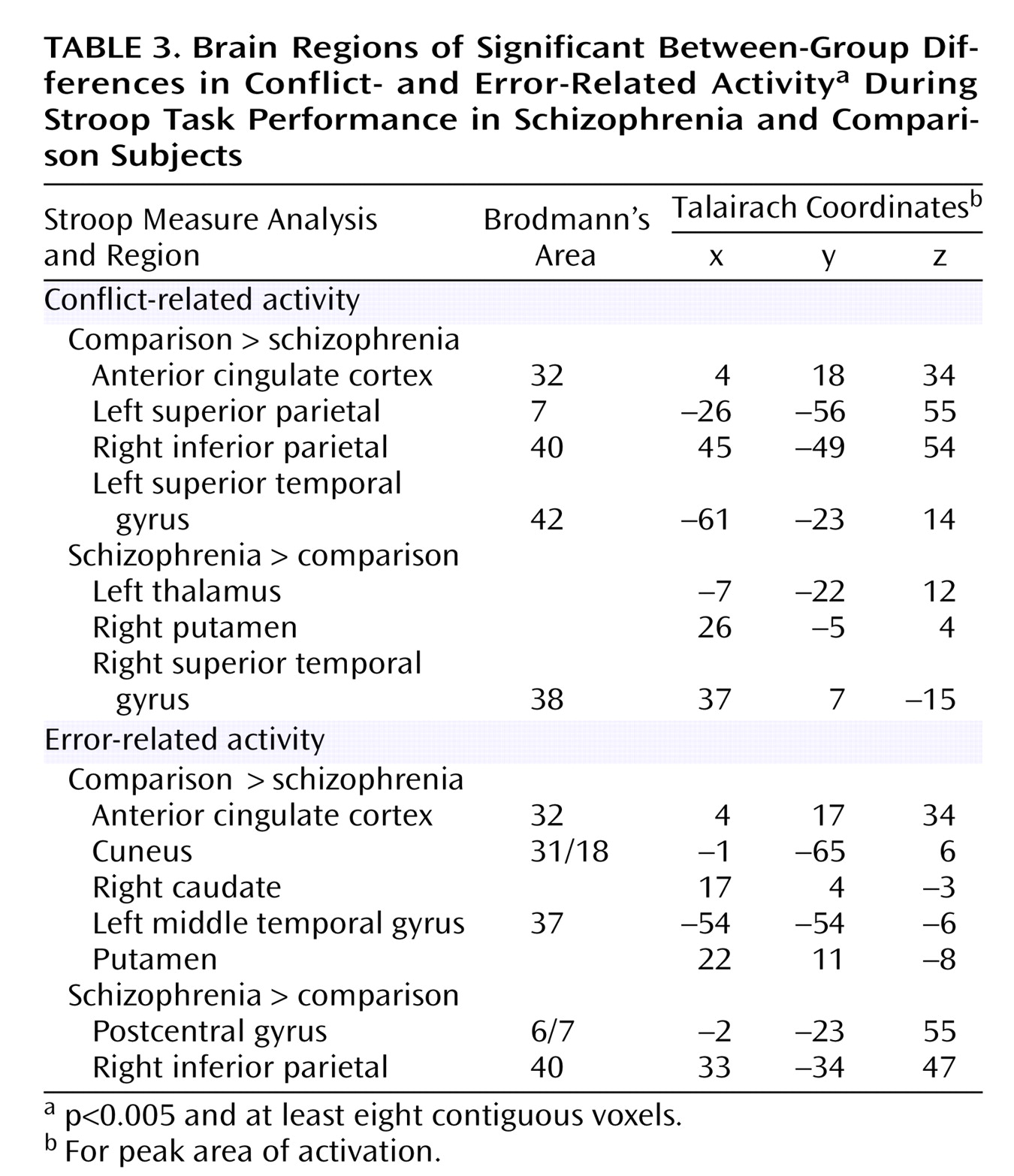
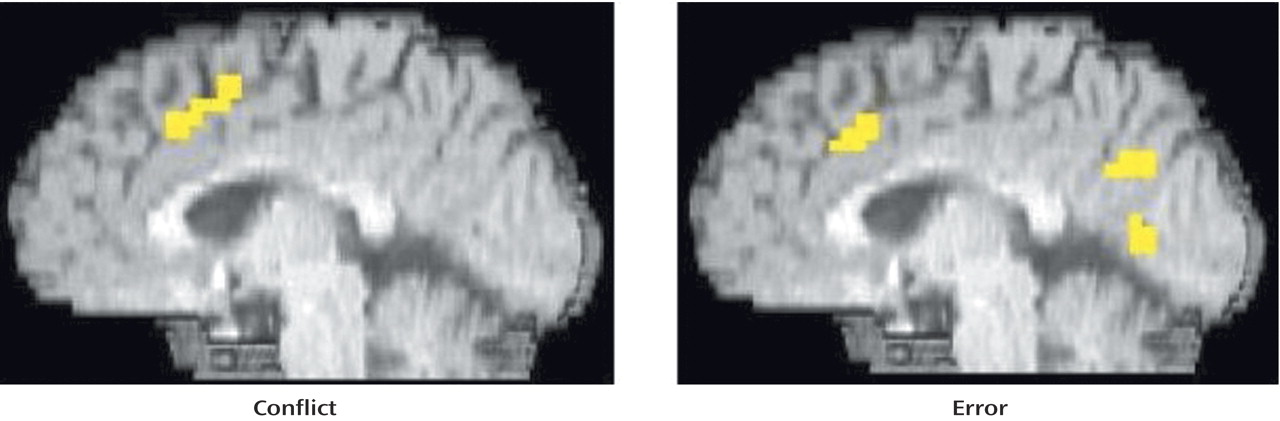
As can be seen in
Figure 3 and
Table 3, comparison subjects exhibited both greater conflict-related activity (number of voxels=22) and error-related activity (number of voxels=29) than people with schizophrenia in a largely overlapping region of the anterior cingulate cortex. Using the anterior cingulate cortex region in which there was a significant between-group difference in conflict-related activity as a region of interest, we found significantly reduced error-related activity in the schizophrenia group. Similarly, we found significantly decreased conflict-related activity in schizophrenia subjects in the region with a between-group difference in error-related anterior cingulate cortex activity. As can be seen in
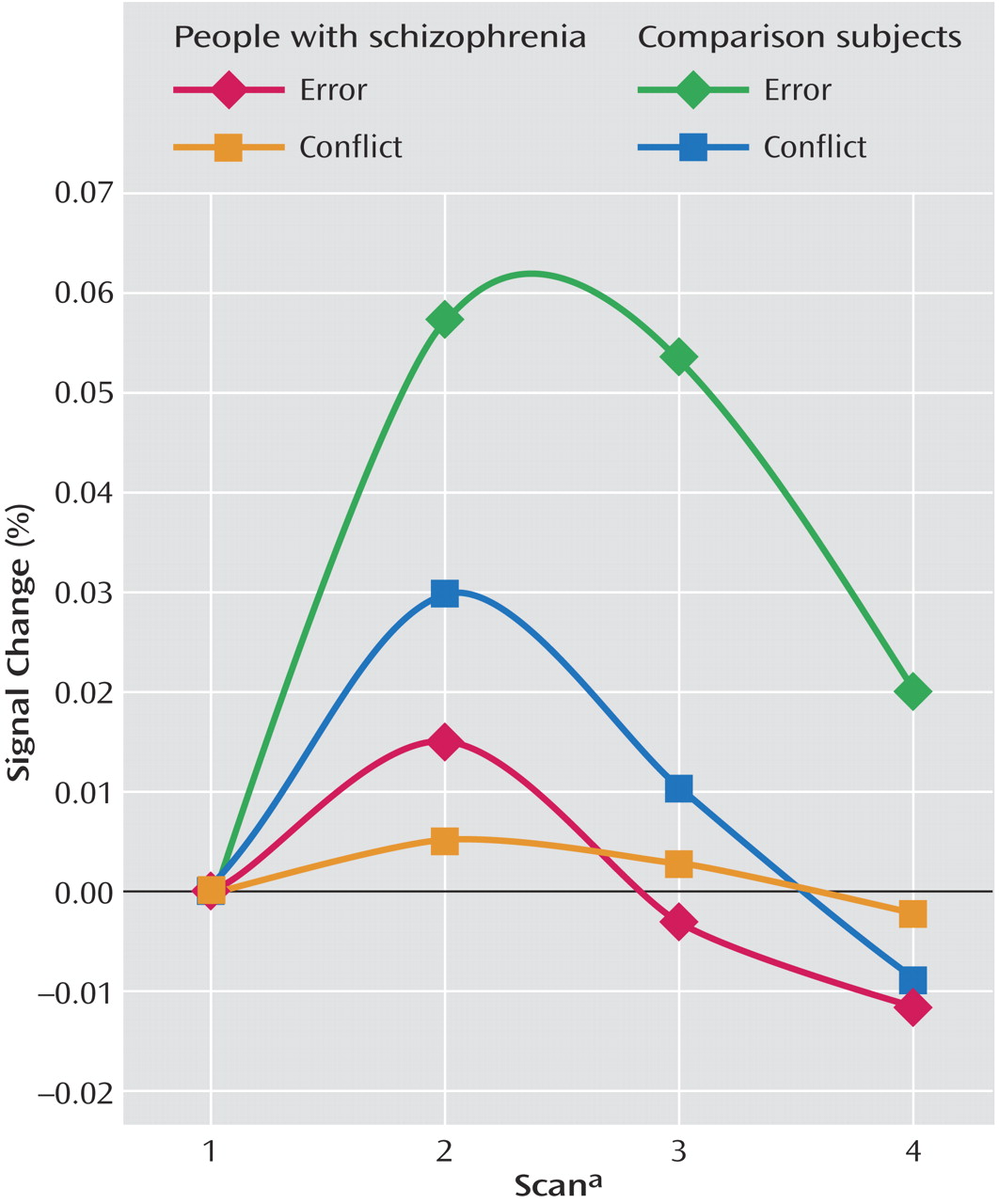
Figure 4, relative to comparison subjects, people with schizophrenia had a comparable time of activation onset in the anterior cingulate cortex, but their amplitude of response was greatly reduced. Thus, people with schizophrenia exhibited reduced conflict- and error-related activity in the same overlapping region of the anterior cingulate cortex.
Discussion
Previous studies have reported reduced anterior cingulate cortex activity in people with schizophrenia performing a variety of tasks, including during the commission of errors and during correct performance of complex tasks likely to elicit conflict
(3–
7). This is the first study to concurrently examine and report decreased conflict- and error-related activity in the anterior cingulate cortex in the illness. The decrease in activity was in the same region of the anterior cingulate cortex for both conflict and errors. The area of the anterior cingulate cortex exhibiting decreased activity in people with schizophrenia corresponds closely to a region identified in a review of previous imaging studies as exhibiting maximum anterior cingulate cortex conflict activity during manual response tasks (Talairach coordinates: x=3, y=19, z=35)
(14). Moreover, healthy participants in the present study tended to exhibit greater trial-to-trial performance adjustments than the schizophrenia subjects, who exhibited a near absence of post-error and post-conflict adjustments. These results are consistent with the hypothesis that anterior cingulate cortex dysfunction in schizophrenia, at least in part, reflects impairment in conflict monitoring that supports trial-by-trial adjustments in cognitive control. It further suggests that poor anterior cingulate cortex conflict monitoring might play a role in the deficits in cognitive regulation exhibited by people with schizophrenia.
Consistent with the conflict theory of anterior cingulate cortex-based performance monitoring and with another recent study that used the Stroop task
(17), there was little difference between the area of the anterior cingulate cortex activated by conflict and that activated by errors in the comparison subjects. This contrasts with some reports that the area of the anterior cingulate cortex activated by errors is more anterior and inferior to the area activated by conflict
(25–
27) in go/no go tasks. An important difference between conflict and error trials in go/no go tasks is that on correct conflict trials people withhold a response but on error trials individuals execute one. In contrast, during the Stroop, correct conflict and error trials both involve overtly responding, perhaps explaining why conflict and error regions during the Stroop are more similar than on go/no go tasks. Moreover, the presumed error-specific region identified in these studies does exhibit conflict activity when considered in the context of the broader literature. The error-related maxima identified by Braver et al. (x=–1, y=21, z=27)
(25) and by Ullsperger and von Cramon (x=7, y=19, z=30)
(26) clearly fall within the area activated by conflict tasks (
Figure 1,
Table 1,
Table 2, and
Table 3) as identified in a review of anterior cingulate cortex activity
(14).
On the surface, the present results appear inconsistent with those of Laurens et al.
(28) who reported decreased rostral anterior cingulate cortex activity but not caudal anterior cingulate cortex activity during errors in schizophrenia. The area of reduced error-related activity reported by Laurens et al. (x=–8, y=44, z=12) is quite distinct from the anterior cingulate cortex activity in the current study and from the putative error distinct region identified by others
(25–
27). As suggested by Laurens et al., the region they identified might reflect emotional responses to errors rather than conflict-based performance monitoring, which appears disrupted in the present study. In similar event-related potential research, we have found different event-related potential error components localized to distinct areas of the anterior cingulate cortex, with one component (hypothesized to reflect conflict-related activity) associated with the caudal anterior cingulate cortex and another (hypothesized to reflect an emotional response to committing an error) being associated with the rostral anterior cingulate cortex
(29). In the current study, neither schizophrenia nor comparison subjects exhibited significant rostral anterior cingulate cortex activity. Therefore, our results do not address whether patients might have an additional emotion processing deficit in the rostral anterior cingulate cortex, which we consider to be an interesting possibility that merits further research.
A second difference between the Laurens et al. study and the present one is that Laurens et al. did not report group differences in the caudal anterior cingulate cortex region associated with conflict processing. In that study, comparison subjects did not exhibit significant caudal anterior cingulate cortex activity in the conflict contrast, rendering a test of this function in patients impossible. The present study is, to our knowledge, the first to concurrently examine both conflict- and error-related activity in people with schizophrenia, finding both decreased conflict- and error-related activity in the same anterior cingulate cortex region.
An important caveat to the current results is that all patients were chronic outpatients taking (mostly atypical) antipsychotic medication. Dopamine blocking drugs may effect the functioning of the anterior cingulate cortex
(30). Thus, it will be important in future studies to examine anterior cingulate cortex functional activity in unmedicated schizophrenia subjects.
According to conflict theory, anterior cingulate cortex conflict monitoring generates a signal that results in greater recruitment of cognitive control
(2). Thus, conflict monitoring should result in behavioral adjustments in performance. In the current study, healthy participants exhibited both significant post-conflict adjustments and post-error slowing. In contrast, schizophrenia subjects exhibited a near absence of these adjustments. This is the first study to report a lack of post-conflict adjustment in people with schizophrenia. Previous research has been inconsistent with regard to whether people with schizophrenia exhibit reduced post-error slowing
(7,
8,
10,
11). It is possible that not measuring post-error slowing separately for congruent and incongruent trials could account for these inconsistent results. The current results suggest that reduced anterior cingulate cortex conflict monitoring in schizophrenia could result in impaired recruitment of prefrontal cortex-based cognitive control. Thus, the current results suggest that poor anterior cingulate cortex conflict monitoring could have a broad influence on the cognitive difficulties experienced by people with schizophrenia.
Given previous research associating cognitive control deficits with symptoms
(31), one issue for future research is to examine associations between anterior cingulate cortex conflict monitoring and symptoms of schizophrenia. For example, psychotic symptoms have been attributed to a deficit in monitoring
(32). At the same time, given the role of the anterior cingulate cortex in cognitive control
(2), it is possible that anterior cingulate cortex dysfunction could contribute to disorganization. Another important implication of the current results is for the relationship between impaired cognition and functional outcome in schizophrenia
(33). Anterior cingulate cortex conflict monitoring has been found to contribute to a number of cognitive domains, such as learning and memory
(14). Hence, disturbances in conflict monitoring might contribute to many cognitive deficits found to be predictive of functional outcome in the disorder. We would suggest that the relationship between anterior cingulate cortex function in schizophrenia to medication treatment and functional outcome should be the subject of further investigation.